Pelvic inflammatory disease (PID) is an infection of the upper vaginal tract in women that can be subclinical or severe, affecting any or all of the uterus, fallopian tubes, and ovaries. Tuboovarian abscess (TOA) is a common consequence of PID. The purpose of this study is to compare the demographic features, symptoms, clinical findings, and treatment techniques of patients hospitalized with the diagnosis of PID and TOA, to assess their response to treatment protocols and therapy, and to evaluate the development of recurrence.
The study, a descriptive retrospective cohort analysis, included 318 patients with PID and TOA who were admitted to the Gynecology Clinic of the University of Health Sciences Etlik Zubeyde Hanim Women's Health Training and Research Hospital between January 1, 2016 and August 1, 2019.
In terms of age, gravida, parity, BMI, educational background, profession, comorbidities, and previous PID attacks, there were no statistically significant differences between the PID and TOA groups. Among birth control techniques, the TOA group had a higher rate of intrauterine device usage, infection indicators (white blood cell, C-reactive protein, and erythrocyte sedimentation rate), and smoking. The PID group had a significantly greater prior history of uterus surgery. While the patients' symptoms and length of complaints were comparable in both groups, the duration of antibiotherapy was statistically longer in the TOA group.
The incidence of active smoking and the mean hospitalization day were found to be considerably higher in the TOA group, which was consistent with the literature in our series. In the literature, the most risky period for the development of PID was stated to be 21 days after the insertion of an intrauterine device, whereas in our study, it was determined that 20.8 percent of the acute PID group and 47.7 percent of the TOA group had an intrauterine device, and that these patients had long-term use of intrauterine devices. Although there is evidence that laparoscopic surgery may be performed successfully in TOA surgery, most surgeons prefer laparotomy. In our series, the rate of minimally invasive surgery was 88 percent. The study's strength is that it is the biggest single-center cohort investigation of hospitalized PID and TOA patients, comparing demographic and physical features, symptoms and signs, clinical course, treatment procedures, and follow-up methods. There have been few studies in this area, and the number of cases is fewer than in our series. Our study's limitations include the fact that it is retrospective, and not all diagnoses can be validated by culture.
Pelvic inflammatory disease, Tubaovarian abscess, Risk factors, Medical therapy, Surgical treatment, Recurrence
BMI: Body Mass Index; CDC: Centers for Disease Control and Prevention; CRP: C-Reactive Protein; ESR: Eritrocyte Sedimentation Rate; IUD: Intra Uterin Device; PID: Pelvic Inflammatory Disease; TOA: Tuba Ovarian Abscess; WBC: White Blood Cell
PID is a subclinical or acute infection of the upper genital tract in women, affecting some or all of the uterus, fallopian tubes, and ovaries. The image might also include the involvement of nearby pelvic organs. Endometritis, salpingitis, oophoritis, perihepatitis, and tuboovarian abscess are all potential risks of PID. The tuboovarian abscess (TOA) is a common consequence of PID. The inflammatory process might lead to rupture, peritonitis, or a potentially fatal septic infection [1].
According to US data, roughly 90,000 people applied for PID in the previous 10 years, but because it is a clinical diagnosis, the exact number of patients is unknown [2]. The rate of TOA has risen in recent years, however with the introduction of contemporary treatment methods, the rate of TOA-related mortality has decreased from 50% to zero [3]. Although the mortality rate has been found to decrease, the risk of morbidity and treatment costs rise with delayed identification of PID. As a result, PID is one of the gynecological diagnoses that should be suspected at a low level, particularly in women in their reproductive years who report with severe pelvic pain. It should also be considered in individuals who have infertility, ectopic pregnancy, or chronic pelvic pain but have no prior history of PID [4].
Broad-spectrum empirical antibiotics yield more than 90% microbiological and clinical improvement in meta-analyses for the treatment of PID [5]. Broad-spectrum parenteral antibiotics are used to treat severe PID that necessitates hospitalization. Because of the severity of the infection, the possibility of abscess rupture, the risk of sepsis, and a suspect diagnosis, TOA requires close surveillance and, as a result, hospitalization. Successful antibiotic therapy and antibiotic penetration into the abscess wall have changed the primary treatment of TOA, and solely antibiotic therapy has been demonstrated to be roughly 70% effective depending on the features of the abscess [6]. Unresponsiveness to antibiotic treatment, abscess rupture, and/or unstable hemodynamics may need surgical intervention. Although both laparoscopic surgery and laparotomy are options for TOA surgery, the majority of surgeons choose for laparotomy [7].
The major goal of this study is to compare the two groups by examining demographic features, symptoms, clinical findings, risk factors, and treatment methods among inpatients with PID and TOA diagnoses. Our secondary goal is to figure out how patients respond to therapy and what happens after they leave the hospital, as well as if there are any recurrences.
The study was designed as an observational retrospective cohort study and began after receiving approval from the Clinical Ethics Committee of the Saglik Bilimleri University Etlik Zubeyde Hanim Gynecology Training and Research Hospital (dated 28/11/2019 and numbered 18/2). 318 patients with PID and TOA diagnoses who were hospitalized at Etlik Zubeyde Hanm Gynecology Training and Research Hospital Gynecology Clinic and followed up and treated (medical and surgical) were included in the research between January 1, 2016 and August 1, 2019. Patients who were brought to our hospital for follow-up and treatment with the diagnoses of PID and TOA, but whose diagnoses changed after the exams, and who began treatment at our hospital but wanted to continue treatment in an external facility, were excluded from the research.
The demographic features, symptoms and clinical findings, risk factors, and treatment modalities of patients admitted to the hospital with PID and TOA diagnoses were studied. Second, therapeutic response as well as post-discharge processes and recurrences were investigated.
Patients' demographics, BMIs, comorbidities, previous surgery histories, pelvic inflammatory disease histories, contraception methods, complaints at the time of admission, and treatment histories prior to hospitalization were scanned and recorded retrospectively from the hospital information system and patient files. Full blood count parameters (hemoglobin, white blood cell count), C-reactive protein, erythrocyte sedimentation rate, CA-125, and complete urinalysis values were recorded, as well as physical examination findings, laboratory values, and pelvic imaging results of the patients. The clinical results of the patients at the time of admission and the control exams completed within the 48-72 hour were compared to assess the patients' reactions to the therapies applied. The effects of medical and surgical treatment options, as well as the continuation of empirical antibiotic medication and treatment changes, were studied.
The results of the PID ultrasonography were classified as (I) Normal, (II) Pelvic fluid, or (III) Hydrosalpenx. TOA was found to be a complex adnexal mass with uneven and thick walls, dense content, and the ovary and tuba could not be seen individually on ultrasonography. Its longest dimension was measured. TOA was diagnosed intraoperatively based on the patients' clinical symptoms and concomitant pelvic imaging results, or during procedures for causes such as acute abdominal and pelvic mass. The greatest sizes, localizations, unilateral or bilateral nature of the masses, as well as concomitant uterine and ovarian diseases, were also noted.
Patients' treatment regimens were categorised, and preoperative clinical data, laboratory values, and ultrasonographic results were documented for those who underwent surgery. Methods of surgery, further procedures, intraoperative and postoperative problems, pathology findings, and abscess cultures were all investigated. All patients' hospitalization days and clinical data before, during, and after discharge were assessed for the occurrence of recurrence and documented.
Patients diagnosed with TOA were given gentamicin and clindamycin at the time of admission, according to our clinic's standard treatment protocol. Clinical and ultrasonographic findings were evaluated 48-72 hours later, and the treatment regimen was changed if the patients were unresponsive or did not respond to the treatment, while those who did respond were kept on their current treatment. The patients were reviewed on the 6th and 10th days of treatment, and it was determined whether to continue with the existing treatment or undergo a surgery. On the 14th day, patients who showed a full response to treatment were released with 14 days of oral antibiotic medication and were summoned for follow-up at regular intervals.
For statistical analysis and computations, IBM SPSS Statistics 22.0 and Microsoft Office - Excel 2016 were utilized. The Kruskal-Wallis Test was used to identify the differences between categorical variables, and central and prevalence measures such as number, percentage, minimum, maximum values, mean, and standard deviation were employed to provide descriptive statistics. The Mann Whitney-U test was used to determine the difference between the normality tests of numerical variables' conformity with normal distribution and the independent variables that did not fit the normal distribution. The student-t test was used to determine the difference between the normality tests of numerical variables' conformity with normal distribution and the independent variables that did not fit the normal distribution. A p value of less than 0.05 (p < 0.05) was judged statistically significant.
Between January 1, 2016, and August 1, 2019, we screened 263560 people who applied to our clinic. Our study comprised 318 hospitalized and followed-up patients with PID and TOA diagnoses, whose data were retrieved and examined retrospectively from the computerized hospital information management system. For whatever reason, no subject was excluded from the study. Table 1 shows the demographic, physical, and clinical characteristics of the patients who took part in the research. The flow chart of the participants included in the study is shown in Figure 1.
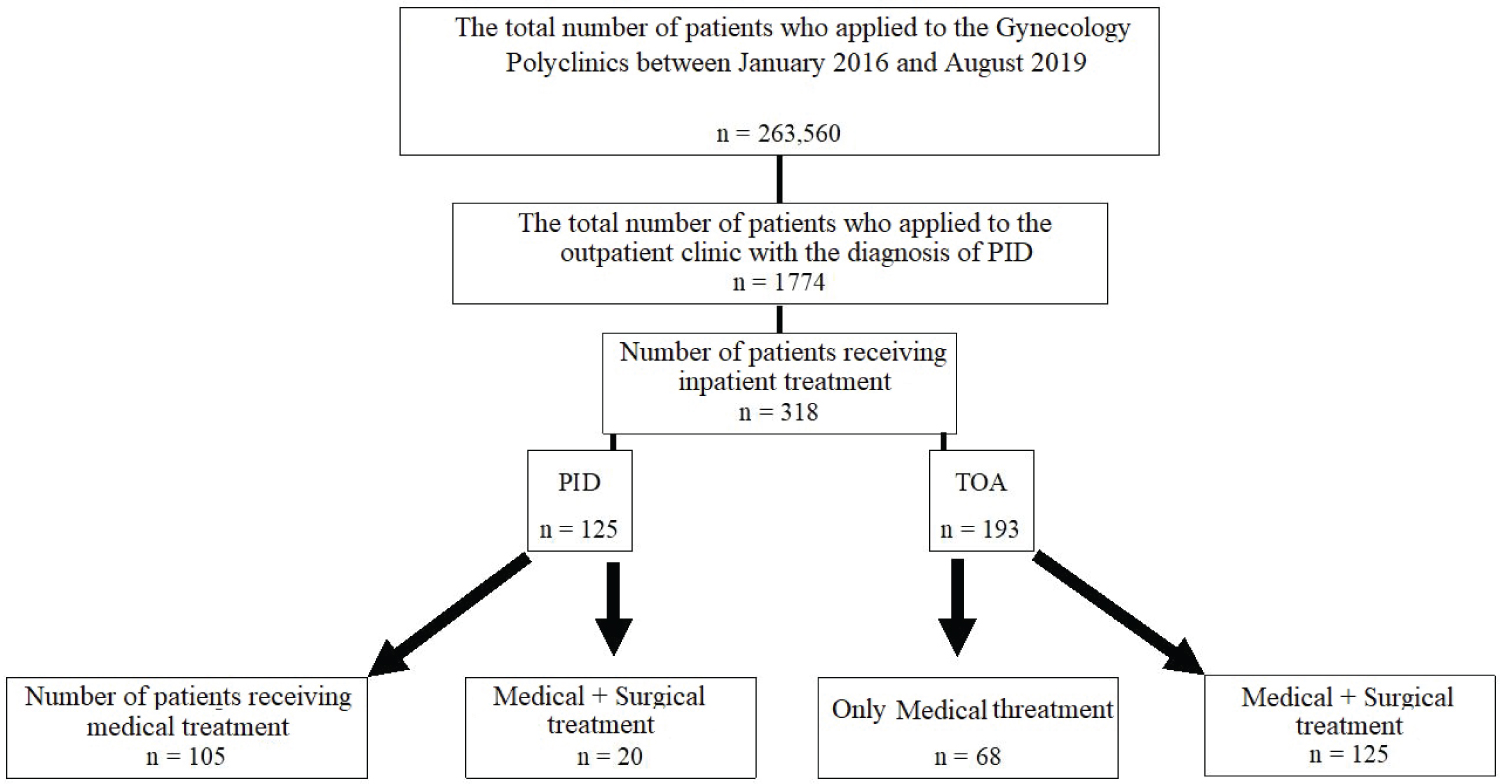 Figure 1: Schematic view of the patients included in the study.
Figure 1: Schematic view of the patients included in the study.
PID: Pelvic Inflammatory Disease; TOA: Tubo-Ovarian Abscess.
View Figure 1
Table 1: Comparison of demographic, physical and clinical characteristics of patients with PID and TOA (n = 318). View Table 1
When the patients' complaints were examined, it was discovered that inguinal pain, discharge, fever, and vaginal spotting were similar in both groups (p > 0.05), and that, despite the fact that the first group was acute PID in terms of duration of complaints, there was no difference in the TOA group (p > 0.05). When the follow-up procedure was analyzed in terms of antibiotic usage length, it was shown that patients diagnosed with TOA got antibiotic medication for a longer period of time (p = 0.022). White blood cell, CRP, and sedimentation levels were substantially higher in the TOA group (p < 0.000) when laboratory findings in terms of infection parameters were compared. While 91.2 percent of PID patients in the USG review revealed no findings, 73 percent of TOA cases revealed unilaterality (Table 2).
Table 2: Evaluation of the complaints and clinical findings of patients diagnosed with PID and TOA (n = 318). View Table 2
In 8.5 percent (n = 27) of the hospitalized patients, intravenous antibiotherapy (gentamicin-clindamycin as first-line therapy) and oral antibiotherapy (doxycycline-metronidazole) were used. Due to insufficient response to medical treatment in the clinical examination and laboratory assessment done 48-72 hours, antibiotherapy was altered in 13.5 percent (n = 43) of the patients in whom medical treatment was commenced. When the patients who had their antibiotic medication changed were assessed, it was discovered that two patients in the acute PID group had their antibiotic therapy changed, and they were released with full recovery. In the TOA group, three patients who received antibiotic therapy were released with full recovery, whereas 18 patients with ruptured TOA and 20 patients with poor response/unresponsiveness to treatment were operated on. When the clinical results of the patients who did not react to therapy were evaluated, 15 patients had bilateral TOA and 5 patients had unilateral TOA. In bilateral TOA, the greatest abscess diameter was 84.3 ± 23.45 (55-140) mm, while in unilateral TOA, it was 66.2 ± 15.3 (40-78) mm.
When patients with TOA follow-up were assessed individually, no significant differences in age or BMI values were detected between those who got just medical treatment and those who had medical + surgical treatment. In terms of past PID, pelvic surgery, intrauterine surgical history, and smoking, which are all risk factors for the development of inflammatory illness (p > 0.05), there was no significant difference. However, although 52.9 percent of patients in the medical + surgical therapy group had an IUD as a form of protection, just 42.6 percent of patients in the medical only treatment group had one (p < 0.000). When the patients' hospitalization laboratory data were analyzed, it was discovered that the surgical treatment group's white blood cell and CRP levels were statistically substantially higher, and the surgery group's duration of hospital stay was greater, but not statistically significant. It was discovered that patients in the medical treatment group had a considerably greater recurrence rate after discharge than those in the surgical treatment group. Patients who received both medical and surgical treatment had considerably bigger abscesses.
Table 3 shows the demographic, physical, and clinical features of individuals with TOA, as well as therapy options.
Table 3: Comparison of demographic, physical and clinical characteristics and treatment modalities of patients with TOA (n = 193). View Table 3
When patients with TOA had medical and surgical therapy, it was discovered that 88 percent (n = 110) of the surgeries were carried out using minimally invasive techniques (transvaginal abscess drainage, laparoscopy). Direct laparotomy was observed to be used in 4% (n = 5) of the cases. The surgeries that began with laparoscopy and ended with laparotomy were found in 8% (n = 10) of the cases.
When all patients were assessed for the development of recurrence, recurrence was found in 10.4% (n = 11) of the PID patients who were released with medical treatment, but only 5% (n = 1) of the PID patients who received surgery. Recurrence rates in TOA cases were 11.7 percent in patients released with medical treatment and 3.2 percent in patients discharged with surgical treatment (p = 0.031). Although there was no difference in recurrence rates between the TOA and PID groups, the surgical groups had lower recurrence rates than those who simply got medical therapy.
PID is an acute inflammatory condition that affects 4% to 12% of women throughout their reproductive years. Early identification and broad-spectrum antibiotic therapy are essential for preventing long-term consequences from PID and lowering treatment costs. Although the prevalence of PID is decreasing, since it is an acute inflammation that may be treated as an outpatient procedure, the incidence of TOA, a serious consequence, is rising. However, modern treatment approaches are proven to have significantly reduced fatality rates [3]. There is no evidence that any of the antibiotic regimens used in the literature are safer or more successful, hence the best treatment strategy is unknown [8]. Although the cause of PID has yet to be identified, certain risk factors have been identified [9].
The great majority of patients with acute symptomatic PID had a poor socio-economic position, according to research investigating demographic features of patients aged 15-44 with acute symptomatic PID. Furthermore, between the ages of 15 and 44, the incidence of acute PID stays consistent in the 25% range, with the maximum incidence occurring between the ages of 21.6 percent and 20-24 years [10]. In our study, 68 percent of the patients diagnosed with acute PID were between the ages of 20 and 44, 3.2 percent were between the ages of 20 and 24, and 22% were between the ages of 35 and 39. The age of initiation of sexual activity, which varies by society, and the lack of information on contraceptive methods owing to early age may be the causes of the reported differences in age groups.
Another notable risk factor for PID in the research groups is active smoking history. When individuals with a history of active smoking, which is one of the modifiable risk factors, are compared to patients who have never smoked, the prevalence of acute symptomatic PID and long-term complications rises [11]. In our study, 41.6 percent of the acute PID group had a smoking history, whereas 53.9 percent of the TOA group had active smoking, which was consistent with the literature. Furthermore, when the association between treatment methods and smoking was examined, it was shown that smokers had a greater rate of medical + minimally invasive drainage and/or surgery than non-smokers. Smoking history was assessed as having a detrimental influence on treatment response in these individuals during the evaluation of responsiveness to therapy.
When looking at the other risk variables in the literature, it's clear that the 21 days following the installation of an IUD, which is one of the most often used protection techniques, is the most dangerous period for the development of PID [12]. When it comes to the usage of contraceptive techniques in our society, it is well known that the coitus interruptus method (withdrawal method) is the most popular, while the IUD is the second most popular [13]. Patients with TOA who had medical + surgical therapy had a greater prevalence of previous pelvic surgery or intrauterine intervention than patients who received medical treatment alone, according to the literature [14]. In our study, we discovered that 20.8 percent of patients with acute PID and 47.7% of patients with TOA had an IUD, and that these patients had been using it for a long time (7.1 ± 5.2 years).
The most prevalent complaints for PID, according to the literature, are inguinal pain, discharge, and fever, however these are not specific problems [5]. In our series, we found that the complaints of patients in the acute PID and TOA groups were comparable, with inguinal pain being the most prevalent symptom (acute PID, 96.8 percent; TOA, 97.9 percent). Dysuria and urinary tract infection were also shown to be associated with both groups at a rate of 35-38 percent. Only 7.5 percent of the patients had a fever (body temperature of 38 ℃ or above, measured twice). It was speculated that this predicament was caused by the fact that some of the patients had undergone antibiotic medication prior to being admitted to the hospital.
In PID, sensitivity to cervical/uterine/adnexal motions during pelvic examination is a key finding. Although the sensitivity and specificity of white blood cell count and C-reactive protein for PID are poor, they can be useful in determining the degree of inflammation and treatment response [2]. While leukocytosis is present in a tiny percentage of acute PIDs, there is evidence that it is more common in TOA [6]. There were diffuse tenderness findings in the pelvic exams done in our clinic, with adnexal soreness predominating in both groups (63.2 percent in acute PID group patients, 77.7 percent in TOA group patients). WBC, ESR, and CRP levels were substantially greater in the TOA group than in the acute PID group in our study. Furthermore, when all TOA cases were divided into groups who received just medical care and those who underwent surgery, the group that underwent surgery had considerably greater leukocytosis and CRP levels.
The duration of hospitalization in the TOA group was considerably longer in research comparing simple acute PID and TOA patients in the literature [15]. While the mean hospital stay in the acute PID group was 7.21 ± 4.0 days, it was 13.8 ± 5.0 days in the TOA group, and the hospitalization days in the TOA group were considerably greater.
The efficiency of parenteral regimens in the treatment of PID was established in 2015 CDC guidelines, which also stressed the need for a treatment regimen with a broad range anaerobic scope [2]. In our own clinical practice, we use the CDC guideline in conjunction with a clinical protocol developed from our own clinical experience. Our patients were given a 140 mg IV loading dosage of gentamicin, followed by a maintenance dose of 80 mg IV 3 × 1 + clindamycin 900 mg IV 3 × 1. Patients are re-evaluated using clinical, laboratory, and ultrasonographic data at the 48-72nd hour of therapy. While the present therapies for patients who respond to treatment are continued, the antibiotic medication is modified (ceftriaxone 1 gr 2 × 1 IV + metronidazole 500 mg 3 × 1 IV) in situations where the treatment does not work. Following the end of IV therapy, patients with a full response to the treatment are released by moving to oral doxycycline 100 mg 2 × 1 + ornidazole 500 mg 3 × 1 (+14 days) regimens. At the 72nd hour evaluation, 13.5 percent of the patients who were started on parenteral therapy were resistant to treatment, and antibiotics were changed. When it comes to evaluating patients who have had their antibiotic medication changed, the TOA group stands out. It was discovered that 38 of the 41 TOA patients (92.7%) received surgery. As may be deduced from this information, patients with complex TOA who require antibiotic modifications may be referred to surgery, and patients should be advised of this possibility.
Although there is evidence that laparoscopic surgery may be performed effectively in TOA surgery, the surgical technique and procedure used is primarily dependent on the surgeon's ability, and most surgeons prefer laparotomy [7]. The rate of laparotomy has been recorded as high as 66 percent in various studies [7]. All patients in the TOA group were operated and underwent open surgery (total abdominal hysterectomy + bilateral salpingectomy) in the research done by Sordia-Hernández, et al., in which they analyzed a total of 52 patients (26 patients with PID, 26 patients with TOA) [16]. Patients contemplating the benefits of laparoscopy will benefit greatly by combining open surgery expertise with a minimally invasive surgical method. Our minimally invasive surgery rate was 88 percent in our series, with laparoscopic surgery accounting for 83.2 percent of the total. Our conversion rate to laparotomy after laparoscopy was 4%, but this statistic has now been zeroed owing to increased experience, particularly in the previous two years.
Because of severe PID that does not respond to conventional therapy, surgery is seldom indicated in acute PID patients [17]. When looking at the complications of PID in the literature, recurrence is the most common, with a rate of 15% recorded following PID [18]. In our study, 65 percent of the patients with acute PID who had medical + surgical therapy had hydrosalpenx that did not respond to treatment, and 35 percent developed salpingitis, with just one patient experiencing recurrence. Recurrence was seen in four individuals in the TOA group who had undergone medical + surgical therapy; three of these patients had bilateral salpingectomy and one had unilateral salpingectomy. Recurrence was seen in 10.4% of patients in the acute PID group who were discharged with medical therapy, and 5% of patients who got medical + surgical treatment in our research. Recurrence was seen in 11.7 percent of TOA patients who were discharged with medical therapy and 3.2 percent of those who were discharged with medical and surgical treatment. When compared to other groups, the recurrence rate of individuals who had medical + surgical therapy is relatively low. It should be noted that in treatment modalities that include surgical therapy and drainage, the chance of recurrence is reduced. Patients receiving therapy should be informed that recurrence rates will be high if solely antibiotic treatment is used.
Our study's limitations must be considered. The first and most serious flaw is that it is designed retrospective. Another key issue is that culture could not confirm all diagnosis.
As a result, it's critical to be cautious while following up on all PID cases and to provide the necessary therapy in a timely manner. Another crucial difficulty is correctly diagnosing TOA, which is a critical clinical picture, and not delaying therapy. It's important to understand the need of rigorous case monitoring throughout follow-up, and it's also important to remember that the procedure can be a bit more challenging, especially in instances with high infection indicators. It's vital to note that rupture can occur in TOA instances with strong infection indicators. It is beneficial to advise the patient that surgical drainage may be necessary in situations with high TOA test results, as this will aid in the clinical healing process and lower the risk of recurrence.
The author thanks all physicians and other staff of the Saglik Bilimleri University Etlik Zubeyde Hanim Gynecology Training and Research Hospital. The author states that she has no competing interests.
The authors declare no conflicts of interest.
All authors contributed to the study conception and design. Busra Kanyildiz was in charge of material preparation, data collection, analysis, and the initial draft of the paper, and the author provided feedback on prior drafts. The author read and approved the final manuscript.