Objectives: Fluoroquinolones are frequently prescribed due to their wide range of antibacterial effects, ease of administration, and widespread availability. However, they are also the second most frequent antibiotics associated with drug allergies. Although it is effectiveness, the potential for causing severe allergic reactions may restrict the available treatment options. Mesenchymal stem cells have the potential to be used as therapeutic agents because of their ability to modulate the immune system and reduce inflammation. They have the potential to be candidates for the treatment of drug-induced hypersensitivity as they release bioactive substances that regulate the activity of immune cells. In this study, allogeneic mesenchymal stem cells were utilized as a therapeutic approach to address the patient's hypersensitivity to ciprofloxacin.
Case Summary: Following cellular treatment, the patient's eosinophil cationic protein levels returned to a normal range, and there was a significant decrease in ciprofloxacin-specific IgE antibodies from 560 pg/ml to 50 pg/ml. No adverse effects were seen throughout the treatment.
Practice Implications: According to our findings, mesenchymal stem cells have the ability to regulate the immune response in cases of drug-induced hypersensitivity. Additional research is necessary to comprehend the impact of cell treatments on the immune system.
Drug-induced hypersensitivity, Mesenchymal stem cells, Cell therapy
Fluoroquinolones, a type of antibiotic, are frequently prescribed due to their wide-ranging antibacterial effects, convenient pharmacokinetic features, ability to be taken orally or intravenously, and global availability [1]. They possess the capability to impede DNA replication in both Gram-positive and Gram-negative bacteria, enabling them to effectively combat microbial infections [2]. As a result, they are employed to address a diverse range of infections, such as urinary, respiratory, gastrointestinal, and intraabdominal infections, in addition to sexually transmitted illnesses [3]. At present, the fluoroquinolones that are most often prescribed are ciprofloxacin, levofloxacin, and moxifloxacin.
However, fluoroquinolone usage has been associated with adverse events (AEs), including hypersensitivity responses (HRs), similar to other medicines. They rank as the second most prevalent category of antibiotics linked to drug allergies, behind beta-lactams [1]. Due to the incomplete understanding of the mechanism behind fluoroquinolone-induced HRs, the actual occurrence rate of these events is still unclear. In certain patients who are hypersensitive to ciprofloxacin, which can lead to severe allergic reactions, such as urticaria, angioedema, or anaphylaxis [4], the use of an alternative therapeutic methods is required. This situation restricts the treatment options for such patients.
Mesenchymal stem cells (MSCs) are being acknowledged as a promising therapeutic option due to their immunomodulatory and anti-inflammatory properties [5,6]. MSCs, derived from different tissues such as bone marrow, adipose tissue, and umbilical cord, have the capacity to regulate immune responses, facilitate tissue regeneration, and decrease inflammation. Their immuno modulatory effects are primarily achieved through the secretion of cytokines, interleukins and growth factors that regulate the function of different immune cells. This makes them a potential option for treating severe allergic reactions, such as drug-induced hypersensitivity [7].
This case report highlights an effective application of MSC therapy in managing a severe allergic reaction to ciprofloxacin. Our study demonstrates the potential of MSCs as a novel therapeutic approach for drug allergies, highlighting their capacity to regulate the immune system and the encouraging clinical outcomes observed in our patient.
The human umbilical cord derived MSCs (hUC-MSCs) were obtained from the Liv Med Cell Good Manufacturing Practice (GMP) facility located in Istanbul, Turkey. The umbilical cord was acquired from a single donor after obtaining their informed consent, in accordance with the authorization of the institutional regulatory board (Liv Med Cell). The umbilical cord, obtained postpartum, was procured from a donor who had undergone a full-term pregnancy. All cell therapy applications were performed after informed consent of the patient.
The umbilical cord was washed with phosphate-buffered saline (Invitrogen/Gibco, Paisley, UK). Following the extraction of blood vessels, the tissue was meticulously divided into explants with dimensions ranging from 5 to 10 mm 3 . After obtaining negative results for VDRL, HBV, HCV, and HIV tests, the tissue explants were placed in culture plates and cultured under conditions that mimic the human body (5% CO 2 and 37℃) until cells migrated from the tissue fragments. The cells were cultivated using serum-free MSC NutriStem® XF Medium, which was replaced every 2-3 days. Once the cells achieved a confluency of 80-90%, they were detached using trypsin and sub cultivation was carried out until the completion of the third passage, at which juncture quality control tests were performed to ascertain their attributes (cell count, viability, sterility, toxicity, flow-cytometry and efficacy). The cultivated hUC-MSCs were cryopreserved until the day of the therapy sessions and were subjected to a second series of quality control tests, on the day they were thawed and prepared for administration to the patient. The manufacturing and product testing methods for the production of clinical-grade allogeneic hUC-MSCs were conducted according to GMP standards, which ensured adherence to quality control and quality assurance protocols as required by the Turkish Medicines and Medical Devices Agency.
A female patient in her 30s checked in our facility with chronic skin itching and adverse reactions to multiple medications following a blood transfusion in 2023. The symptoms she presented were erythema and edema on her face and lips. The patient had a medical history of allergic responses, however, her family history did not reveal any significant allergies. She had previous allergic reactions to certain antibiotics and medications, which also caused symptoms like itching, redness, and swelling.
During the initial examination, laboratory tests were conducted to assess immune system function and allergy sensitivity. The test results showed that the levels of anti-thyroglobulin (ATAb: 0.1 IU/ml), IgE (Serum: 25.5 IU/ml), C-reactive protein (CRP: 3 mg/ml), and anti-nuclear antibody (ANA: negative) were all within the normal range. However, the levels of thyroid peroxidase antibody (Anti-TPO: 12.4 IU/ml; normal: < 9 IU/ml) and eosinophil cationic protein (ECP: 26.6 ng/ml; normal: < 24 ng/ml) were elevated. An allergy test was conducted three days later, utilizing the ALEX2 technique to detect molecular reactions to 295 distinct allergens. The test showed a positive result for lobster (Hom g) with a value of 0.59 kUA/l. In addition, various allergens from different categories were found, with IgE concentrations usually below 0.1 kUA/l (total IgE ≤ 100 kU/l). Afterwards, a sensitivity test was performed on ciprofloxacin and clarithromycin tablets. The test results showed a sensitivity of 560 pg/ml for ciprofloxacin tablets, but there was no significant sensitivity observed for clarithromycin tablets (114 pg/ml). On the day test results indicated sensitivity for ciprofloxacin tablets; a three-session intravenous infusion of 140 × 10 6 allogeneic MSCs derived from human umbilical cords (hUC-MSC) was initiated. hUC-MSCs administrations took place with 2 and 3 days intervals respectively. After undergoing cell therapy, the patient was requested to undergo further testing to assess her immune system function and allergic sensitivities.
Three months after the hUC-MSCs administrations, the test results indicated a normalization of ECP levels (15.8 ng/ml), along with elevations in IgE (Serum: 87 IU/ml) and Anti-TPO (16.1 IU/ml), without any change in ATAb levels (0.1 IU/ml). Omalizumab 150 mg/ml was also prescribed to the patient for injection (1 subcutaneous injection of 150 mg every 4 weeks). A second ciprofloxacin sensitivity test was conducted two weeks later, revealing the presence of IgE antibodies specific to ciprofloxacin at a concentration of 100 pg/ml that decreased within the normal range. A repeat test was conducted six months after the hUC-MSCs therapy to assess immune system function and allergy reactions. The results showed a slight rise in ECP levels (19.7 ng/ml) and IgE levels (Serum: 91.1 IU/ml), but a drop in Anti-TPO levels (12.7 IU/ml). The ANA test again yielded negative results. Figure 1 display graphs of all immune system function and allergy test results and their change over 6 months period. Additional tests were performed for the patient 6 months after the operation, including a skin prick test and intradermal tests.
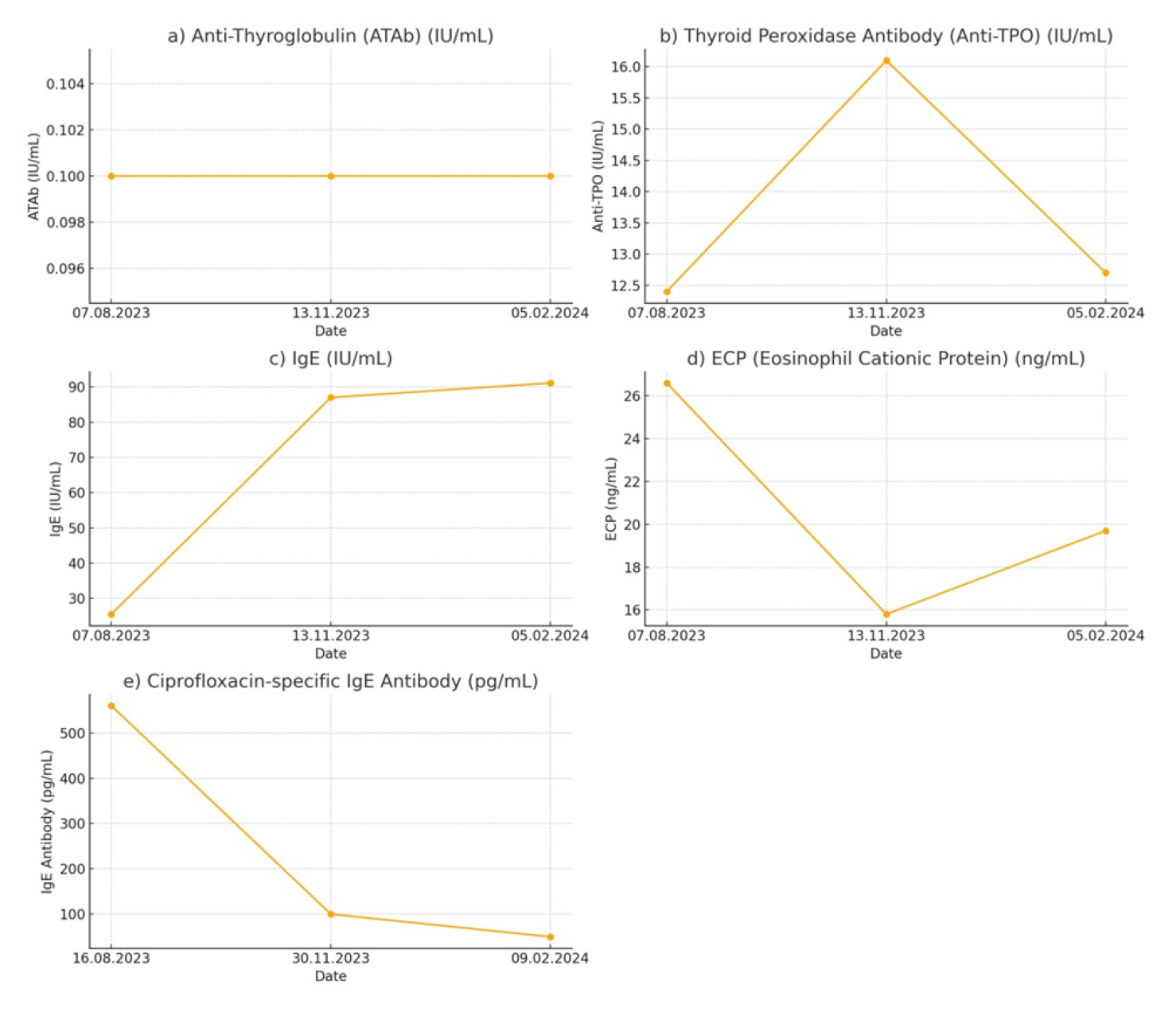 Figure 1: Immune system function and allergy sensitivity test results over 6 months’ time period. The dashed line indicates the time of MSC applications. a) ATAb test results; b) Anti-TPO test results; c) IgE test results; d) ECP test results; e) Ciprofloxacin specific IgE antibody test results.
View Figure 1
Figure 1: Immune system function and allergy sensitivity test results over 6 months’ time period. The dashed line indicates the time of MSC applications. a) ATAb test results; b) Anti-TPO test results; c) IgE test results; d) ECP test results; e) Ciprofloxacin specific IgE antibody test results.
View Figure 1
These tests showed no significant reactions to ciprofloxacin or common food allergens. On the other hand, there were reactions observed to a wide range of allergens including house dust mites, mold, grass pollens, rye pollen, mugwort, wall pellitory, olive tree, birch tree, cat epithelium, and dog epithelium, as well as various other pollens and dust. Another allergy patch test was performed the following day to check for nickel sulfate hexahydrate and cobalt (II) chloride hexahydrate which revealed positive reactions after 72 and 48 hours, respectively. After two days, another sensitivity and allergy test was performed for ciprofloxacin, and there was no evidence of a type I allergy to ciprofloxacin. This was supported by the findings of the ciprofloxacin-specific IgE antibody test, which demonstrated a significant decrease from 560 pg/ml to 50 pg/ml in just 6 months. This result suggests a positive outcome for the immune-regulating effect of hUC-MSC therapy.
Ciprofloxacin, although highly successful in managing bacterial infections, has substantial hazards for people with hypersensitivity responses. This case report describes a female patient in her 30s, who had a previous history of allergic reactions and experienced a severe hypersensitivity reaction to ciprofloxacin. Traditional treatment methods such as antihistamines and corticosteroids, frequently fail to satisfy managing severe allergic responses, which highlights the necessity for developing new therapeutic approaches [8,9].
There is an increasing interest on MSCs for their capacity to modulate the immune system, reduce inflammation, and promote tissue regeneration [6,10,11]. These properties of MSCs make them a promising therapeutic option for a variety of immune-related disorders. Their immune-regulating capabilities have been effectively used in the treatment of conditions such as graft-versus-host disease (GvHD) [12-14], Crohn's disease [15,16], multiple sclerosis [17,18], systemic lupus erythematosus [19,20], rheumatoid arthritis [21,22], and type 1 diabetes [23,24] in the literature. The therapeutic potential of MSCs in these diverse applications highlights their versatility and importance in regulating the immune system.
In the present case, allogeneic hUC-MSCs were utilized to address the patient's severe allergic reaction to ciprofloxacin. The administration of MSC therapy resulted in a significant improvement in the patient's condition. This was shown by the normalization of ECP levels and a significant decrease in ciprofloxacin-specific IgE antibodies from 560 pg/ml to 50 pg/ml over a six-month period. This indicates that MSCs have the ability to efficiently regulate the immune response in instances of drug-induced hypersensitivity. Despite initial increases in anti-TPO levels following cell therapy, they eventually decreased, indicating a possible delayed immunomodulatory effect of MSCs. The IgE levels showed a gradual increase, without any noticeable clinical symptoms, suggesting a complex interaction between multiple immune pathways facilitated by MSCs. Omalizumab, a monoclonal antibody that targets IgE, appears to have enhanced the immunomodulatory effects of hUC-MSCs, leading to a comprehensive approach in treating the patient's hypersensitivity.
Another essential factor in this case report was the safety profile of the cell therapy. The patient exhibited good tolerance to the allogeneic hUC-MSCs infusions, without any documented adverse events. This is consistent with previous research [25,26], which indicates that MSCs are typically safe and well-tolerated in many therapeutic contexts.
This case report emphasizes the promise of MSC therapy as a reliable and secure treatment for severe drug-induced hypersensitivity responses. The significant decrease in ciprofloxacin-specific IgE antibodies and the overall improvement in the patient's clinical condition underscore the potential of MSCs in regulating immunological responses. To our knowledge, this is the first case report of using allogeneic hUC-MSCs to treat ciprofloxacin allergy. Further investigation with larger patient populations is required to gain a more comprehensive understanding of how MSCs influence the immune system and to validate the efficacy of this therapeutic approach in a broader array of clinical scenarios. Integrating MSC therapy into the treatment of medication allergies has the potential to revolutionize the way we manage these allergies, offering new possibilities for patients who have limited treatment options.
Mesenchymal stem cell products were obtained from the Liv Med Cell Good Manufacturing Practice (GMP) facility located in Istanbul, Turkey. The umbilical cord was acquired from a single donor after obtaining their informed consent, in accordance with the authorization of the institutional regulatory board. All cell therapy applications were performed following informed consent of the patient.
All authors hereby provide our consent to participate in the submission of the manuscript titled "Successful Mesenchymal Stem Cell Therapy in a Patient with Ciprofloxacin Sensitivity” to the Clinical Medical Reviews and Case Reports Journal. We understand and agree to the following terms and conditions: We confirm that we have read, reviewed, and contributed to the creation of the manuscript titled "Successful Mesenchymal Stem Cell Therapy in a Patient with Ciprofloxacin Sensitivity". We are aware of its content and have made substantial intellectual contributions to its development. We understand that the submission of this manuscript is being made to the Clinical Medical Reviews and Case Reports Journal and that it is subject to the journal's peer-review process and editorial policies. We acknowledge that we have reviewed and complied with the journal's author guidelines and ethical standards for manuscript submission, including the guidelines on authorship, originality, and conflicts of interest. We understand that the manuscript may be reviewed by independent experts and that revisions may be required before publication. We agree that the corresponding author, Ayberk Akat Ph.D., is authorized to act on our behalf regarding all matters related to the submission, review, and publication of this manuscript, including correspondence with the journal's editorial team. We affirm that the content of this manuscript is original, has not been previously published elsewhere, and is not under consideration for publication in any other journal. We understand that if the manuscript is accepted for publication, it will be made available to the public through the Clinical Medical Reviews and Case Reports Journal and other platforms. We grant the Clinical Medical Reviews and Case Reports Journal the right to use and distribute the manuscript in accordance with its publication policies, including online and in print. We confirm that all co-authors have been informed of and consent to the submission of this manuscript to Clinical Medical Reviews and Case Reports Journal. We acknowledge that any changes to authorship, conflicts of interest, or other relevant information will be promptly communicated to the journal's editorial team. We hereby provide our consent to participate in the submission of this manuscript and confirm that we have reviewed and agree to the terms outlined above.
All authors reviewed the results and approved the final version of the manuscript to be published. All authors hereby give consent for the publication of our personal information in the Clinical Medical Reviews and Case Reports Journal. This information includes, but is not limited to, our name, age, sex, and any other information that is included in the article. We also understand that we are granting the publisher the exclusive right to publish the article in all languages, in whole or in part. We retain the right to use the article for our own purposes, such as teaching, lecturing, and presenting at conferences.
Availability of data and material: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors declare that they have no competing interests.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
RE: Comprehensive evaluation of the patient, analysis of test results and formulation of the treatment plan. Performed MSC therapies, and conducted thorough follow-ups to monitor the patient's progress. His expertise and dedication were pivotal in managing the patient's care throughout the treatment period. AA: Conceived and designed the analysis, analyzed results, drafted the article. EK: Formulation of the cell therapy plan, study conception and design, revised the article critically for important intellectual content.
Not applicable.
Declaration of generative AI and AI-assisted technologies in the writing process: During the preparation of this work the author (s) used AI (Chat GPT) in order to prepare graphs in Figure 1 and to enhance the writing grammatically. After using this tool/service, the author (s) reviewed and edited the content as needed and take (s) full responsibility for the content of the publication.