Moderate aortic stenosis (AS) presents challenges in its management and prognosis. Its diagnosis is based on hemodynamic parameters, but discordance in severity may require additional tests such as stress echocardiography or calcium scoring by computed tomography. Cardiac magnetic resonance imaging (MRI) is useful for evaluating myocardial fibrosis, which is important in prognosis. Other echocardiographic markers, such as Global Longitudinal Strain (GLS) and diastolic dysfunction, also have prognostic implications. Regarding treatment, there is no specific medical treatment for moderate AS. Management focuses on controlling comorbidities and addressing ventricular dysfunction. The decision for aortic valve replacement (AVR) in patients with moderate AS is complex and should consider the presence of symptoms, ventricular function, and other risk factors. Recent studies suggest that early intervention, especially in symptomatic patients or those with signs of ventricular dysfunction, may improve long-term outcomes. However, further research is needed to clarify the role of AVR in moderate AS. Follow-up for these patients should be regular and tailored to their individual clinical profile, considering the possibility of disease progression and the need for intervention.
Moderate aortic stenosis, Aortic valve replacement, Heart failure
Aortic stenosis (AS) is an obstruction of left ventricular (LV) outflow through the aortic valve (AV) due to narrowing of the valve orifice. It is the third most common cardiovascular disease in developed countries after coronary artery disease and hypertension (HTN). Major causes include calcification of a normal tricuspid valve, congenital bicuspid valve with superimposed calcification, congenital aortic valve disease, and rheumatic disease [1-3].
Approximately 10% of patients with AS have heart failure (HF) with reduced left ventricular ejection fraction (LVEF). In this subgroup, only those with severe AS have a Class I indication for aortic valve replacement (AVR) [4]. However, the appropriateness of such management has been questioned in recent publications that report relatively high mortality in this population, raising the question of whether intervention should be considered. Other factors beyond the valve contribute to worse prognosis in these patients, prompting questions about intervention [5].
Moderate AS is defined by hemodynamic parameters such as aortic valve area (AVA) (1.0-1.5 cm 2 ), mean transvalvular gradient (20-40 mmHg), and peak aortic jet velocity (3.0-4.0 m/s) [6,7].
However, about 20-40% of patients with moderate AS present discordant severity measures despite excluding measurement errors. Discordant severity staging can have prognostic and therapeutic implications, requiring additional tests such as dobutamine stress echocardiography (DSE) or computed tomography (CT) AV calcium scoring [8-10].
Discordance observed in moderate AS with normal flow can be due to measurement errors, small size, or reduced arterial compliance [9-11]. Based on the Gorlin formula (and assuming normal cardiac output), an AVA of 1.0 cm 2 corresponds to a mean gradient of 26 mmHg, while an AVA of 0.8 cm 2 to a mean gradient of 40 mmHg, suggesting that guidelines may be inherently inconsistent [12,13].
The first step when encountering discordant patterns is to re-evaluate the accuracy of Doppler echocardiography values. Consider that severity markers may be mitigated in the presence of HTN.
The calculation of the dimensionless index (represented by the ratio of the velocity-time integral (VTI) in the LV outflow tract (LVOT) to the VTI in the AV) eliminates the calculation of the area by cross-sectional measurement of the LVOT.
Indexing AVA by body surface area may be useful in children, adolescents, and short-stature adults. However, it does not necessarily reflect normal AVA in obese patients because AVA does not increase with excess weight [10].
After excluding measurement errors, discordant moderate AS is generally due to low flow states (stroke volume index (SVI) < 35 ml/m 2 or mean transvalvular flow rate < 210 ml/sec). In these cases, AVA may decrease and overestimate AS severity, while peak velocity or mean pressure gradient may decrease, underestimating its severity [10].
Current guidelines have created four categories to better characterize this group. These categories include concordant moderate AS (AVA and mean gradient within guideline ranges) and discordant moderate AS, which are divided into three subcategories:
1) "Classic” low-flow, low-gradient moderate AS with reduced LVEF (< 50%), SVI < 35 ml/m 2 , and mean gradient < 40 mmHg.
2) "Paradoxical” low-flow, low-gradient moderate AS with preserved LVEF (≥ 50%), SVI < 35 ml/m 2 , and mean gradient < 40 mmHg) caused by a small LV size secondary to concentric remodeling that affects ventricular filling and reduces flow, observed in HTN patients. Other factors reducing stroke volume are atrial fibrillation, concomitant mitral or tricuspid regurgitation, and mitral stenosis.
3) Normal-flow, low-gradient moderate AS (LVEF 50%, SVI > 35 ml/m 2 , and mean gradient < 40 mmHg) [7,8].
In cases of discordant severe low-flow, low-gradient AS with AVA < 1.0 cm 2 (severe) and mean gradient < 40 mmHg (moderate), described as "pseudo-severe" AS, a dobutamine stress echocardiogram (DSE) can differentiate the degree of severity by increasing transvalvular flow. Truly severe AS is defined by AVA < 1.0 cm 2 and an increase in mean gradient > 40 mmHg during the test, while pseudo-severe is defined by AVA > 1.0 cm 2 and mean gradient < 40 mmHg.
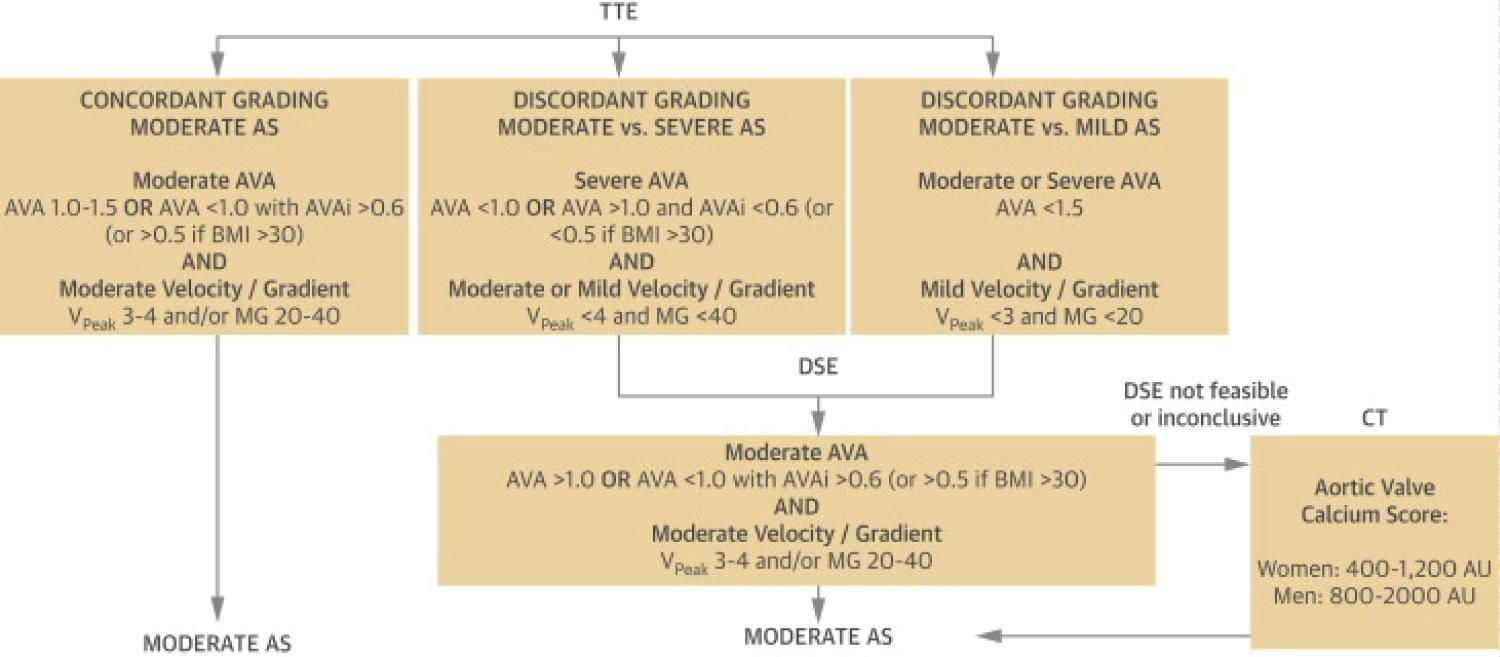
Discordant moderate AS presents with AVA 1.0-1.5 cm 2 and mean gradient < 20 mmHg. DSE can help differentiate between truly moderate and pseudo-moderate AS; however, no studies have evaluated the use of DSE in this group or demonstrated improved risk stratification (Figure 1).
 © 2023 Elsevier. Reprinted with permission from *JACC: Cardiovascular Imaging*, Volume 16, Issue 6, June 2023, Pages 837-855. Available at: http://www.sciencedirect.com/science/journal/1936878X
© 2023 Elsevier. Reprinted with permission from *JACC: Cardiovascular Imaging*, Volume 16, Issue 6, June 2023, Pages 837-855. Available at: http://www.sciencedirect.com/science/journal/1936878X
Figure 1: DSE for confirmation of Moderate AS in case of discordant grading at TTE.
AVA: Aortic Valve Area (in cm2); AVAi: indexed AVA (in cm2/m2); BMI: Body Mass Index (in kg/m2); DSE: Dobutamine Stress Echocardiography; MG: Mean Transaortic Gradient (in mm Hg); VPeak: Peak aortic jet Velocity (in m/s); TTE: Transthoracic Echocardiography.
View Figure 1
In inconclusive cases using DSE (or in those where it is contraindicated), AV calcium scoring by non-contrast cardiac CT can confirm severity. A score between 800-2000 AU in men and 400-1200 AU in women is consistent with moderate AS. Contrast-enhanced AV CT provides additional information and correlates well with AS severity when fibrosis significantly contributes to valve obstruction, which may not be visualized in non-contrast CT. Therefore, moderate AS cannot be excluded in patients with low calcium scores presenting with degenerative valves on echocardiography.
It is important to use flow-independent measures, such as the dimensionless index (DI), to determine severity, calculated by the VTI of the LVOT divided by the VTI of the AV. A DI ≤ 0.25 is consistent with severe AS and is associated with worse outcomes. The risk of events, including cardiovascular death or the need for AVR, increases linearly with a DI < 0.25.
If non-invasive test results are inconclusive, cardiac catheterization (with administration of drugs such as nitroprusside or dobutamine) can determine AS severity [5,10,14-16].
To optimize risk stratification and management in patients with moderate AS, it is important to understand the underlying pathophysiology. Increased LV afterload (secondary to AS) induces LV hypertrophy to reduce wall tension and maintain cardiac output. With the development of hypertrophy, myocardial oxygen demand increases, while coronary flow reserve decreases due to concomitant microvascular dysfunction. Coronary perfusion pressure decreases, and diastolic perfusion time is reduced. This imbalance in oxygen delivery and demand triggers subendocardial ischemia, which combined with direct mechanical forces due to increased afterload and neurohormonal activation, leads to irreversible myocyte degeneration, cell loss, and myocardial fibrosis, ultimately resulting in HF.
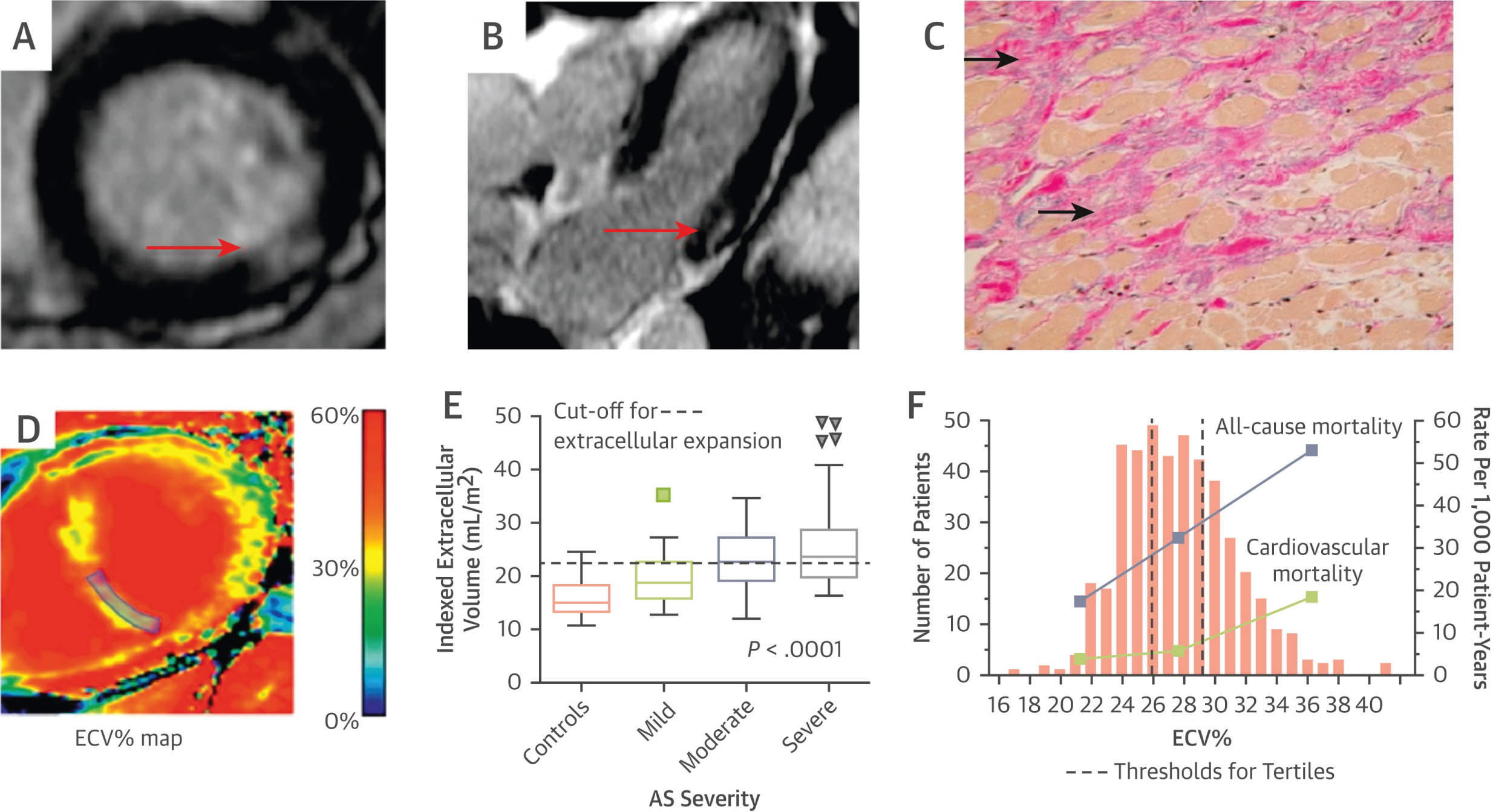
Cardiac MRI provides an adequate characterization of myocardial tissue, allowing non-invasive assessment of LV fibrosis, which can be divided into two types: 1) Reactive interstitial fibrosis and 2) Focal replacement fibrosis. Reactive interstitial fibrosis occurs in the early stages of AS, is diffusely distributed in the LV wall, and can reverse after AVR. A key feature is the expansion of the extracellular space due to excessive collagen deposition. Native T1 values and extracellular volume fraction (ECV) have been validated against histology, showing a correlation with AS severity, LV mass, symptomatic status, and LV function. Among these parameters, the ECV fraction has shown the best reproducibility. Indexing the ECV fraction with LV volume has demonstrated a good correlation with histological fibrosis burden in patients with mild to severe AS [10] (Figure 2).
 © 2023 Elsevier. Reprinted with permission from *JACC: Cardiovascular Imaging*, Volume 16, Issue 6, June 2023, Pages 837-855. Available at: http://www.sciencedirect.com/science/journal/1936878X
© 2023 Elsevier. Reprinted with permission from *JACC: Cardiovascular Imaging*, Volume 16, Issue 6, June 2023, Pages 837-855. Available at: http://www.sciencedirect.com/science/journal/1936878X
Figure 2: Images of Myocardial fibrosis in patients with AS.
Focal area of midwall fibrosis (red arrows) on short- (A) and long-axis (B) views of the inferolateral wall by CMR, corresponding to increased replacement fibrosis in the mid-wall region of the myocardium (solid arrows) on histology using trichrome stain (C); (D) Using T1 mapping techniques (pre- and post-contrast), the ECV% calculates the fraction of myocardium that consists of extracellular space (after incorporating hematocrit) and provides an indirect assessment of the extracellular expansion caused by diffuse interstitial fibrosis; (E) Indexing ECV% to LV volume and body surface area provides good discrimination between healthy and fibrosis-infiltrated myocardium of AS; (F) When ECV% is divided into tertiles in 440 patients with severe AS, both the all-cause (purple squares) and cardiovascular mortality rates (green squares) progressively increased across the tertiles. Not only is ECV% associated with markers of LV decompensation including symptomatic status, it remained as an independent predictor of all-cause mortality on multivariable analysis. (A to C) Adapted with permission from Dweck, et al. [19]; (E) Adapted with permission from Chin, et al. [26]; (F) Graph adapted with permission from Everett, et al. [17]. CMR: Cardiac Magnetic Resonance; ECV%: Extracellular Volume Fraction; LV: Left Ventricular.
View Figure 2
A study by Everett, et al. showed that the ECV fraction provided the most robust prognostic information and was superior to LVEF (each 1% increase in ECV fraction increased the risk of mortality by 10%) [17].
In patients with AS, replacement fibrosis reflects more advanced disease. It is irreversible and presents as a non-infarct pattern (in the mid-wall) on late gadolinium enhancement (LGE) imaging. This is associated with significant LV hypertrophy, reduced LV systolic function, diastolic dysfunction, reduced exercise tolerance, and heart failure [10].
In a multicenter study involving 674 patients with severe AS undergoing AVR, the presence of LGE was a potent and independent predictor of worse outcomes and mortality, correlated in a dose-dependent manner (each 1% increase in LGE increased the risk of mortality by 11% and the risk of cardiovascular mortality by 8%). In a study by Dwerck, et al. involving 143 patients with moderate (40%) or severe (60%) AS, 50% of patients with mid-wall LGE actually had moderate stenosis. Furthermore, more than half of the patients with mid-wall fibrosis who died had moderate AS and would not have been considered for AVR under conventional treatment practices. This raises the question of whether patients with moderate AS and LGE are being referred too late for AVR and continue to have a higher risk of adverse events after AVR [18,19].
Currently, an LVEF ≥ 50% is considered normal; however, recent evidence suggests that a "truly normal" LVEF is closer to 60-65%. Patients with an LVEF ≥ 50% already exhibit subclinical myocardial dysfunction, thus measuring LV systolic function with LVEF seems insufficient to accurately assess early myocardial dysfunction, which can be evaluated through global longitudinal strain (GLS). GLS is useful in risk stratification in patients with moderate AS and preserved LVEF. In patients with AS, LV GLS has been shown to be impaired at an earlier stage than LVEF, and each increase in AS severity (from sclerosis to severe stenosis) is associated with progressive impairment of LV GLS, even with preserved LVEF [10,20].
A study by Zhu, et al. demonstrated that patients with moderate AS and preserved LVEF who exhibited more impaired LV GLS (>−15.2%) had higher mortality rates at 1, 3, and 5 years of follow-up compared to patients with more preserved LV GLS (≤−15.2%): 21%, 35%, and 48% versus 6%, 15%, and 19%, respectively (P < 0.001). In multivariate analysis, each 1% unit impairment in LV GLS was associated with a 19% increased risk of mortality (95% CI: 1.12-1.26; P < 0.001) [21].
LV concentric remodeling, which causes diastolic dysfunction, has also been associated with worse outcomes in patients with moderate AS, showing an independent association with all-cause mortality and the combined endpoint of all-cause mortality and AVR [10,22]. Ito, et al. demonstrated that an elevated E/e′ ratio was present in 54% of patients with moderate AS and was associated with worse outcomes, even among those who were asymptomatic or had an LVEF ≥ 50% [23].
LV diastolic dysfunction can trigger long-term extra-aortic cardiac remodeling, resulting in complications such as elevated left atrial pressures, increased pulmonary arterial pressures, tricuspid regurgitation, and right ventricular dysfunction. These structural changes are associated with adverse outcomes in patients with severe AS [10,24].
Several biomarkers reflecting adaptive changes in AS have been identified, predicting symptoms and clinical events in patients with severe AS and stratifying risks in asymptomatic patients. These markers identify patients with moderate AS who have rapid progression or high cardiovascular risk.
N-terminal pro-B-type natriuretic peptide (NT-proBNP) reflects LV wall stress and correlates with AS severity, symptom onset, and perioperative and long-term mortality. Although high levels are associated with events, there are no studies confirming that early AVR leads to functional improvement or reduced mortality in moderate AS [10,16,25].
In patients with moderate and severe AS, an increase in high-sensitivity troponin I was associated with increased LV mass, fibrosis on CMR, need for AVR, and cardiovascular death, even after adjusting for AS severity and AV calcium score [26].
Capoulade, et al. showed that elevated levels of lipoprotein (a) and oxidized phospholipids correlate with progression and cardiovascular risk in mild to moderate AS, independent of other risk factors, as well as with AVR risk and cardiac death [10,27].
Several recent observational studies have demonstrated that moderate AS is also associated with a considerable risk of adverse cardiovascular events, including death. Unfavorable outcomes have been reported, very similar to patients with severe AS, with 61% experiencing death, hospitalization for heart failure, and AVR requirements within a 4-year period. Additionally, there are conflicts regarding the prognosis in patients with pseudo-severe AS in those with reduced LVEF and low-flow low-gradient AS [8-10,28].
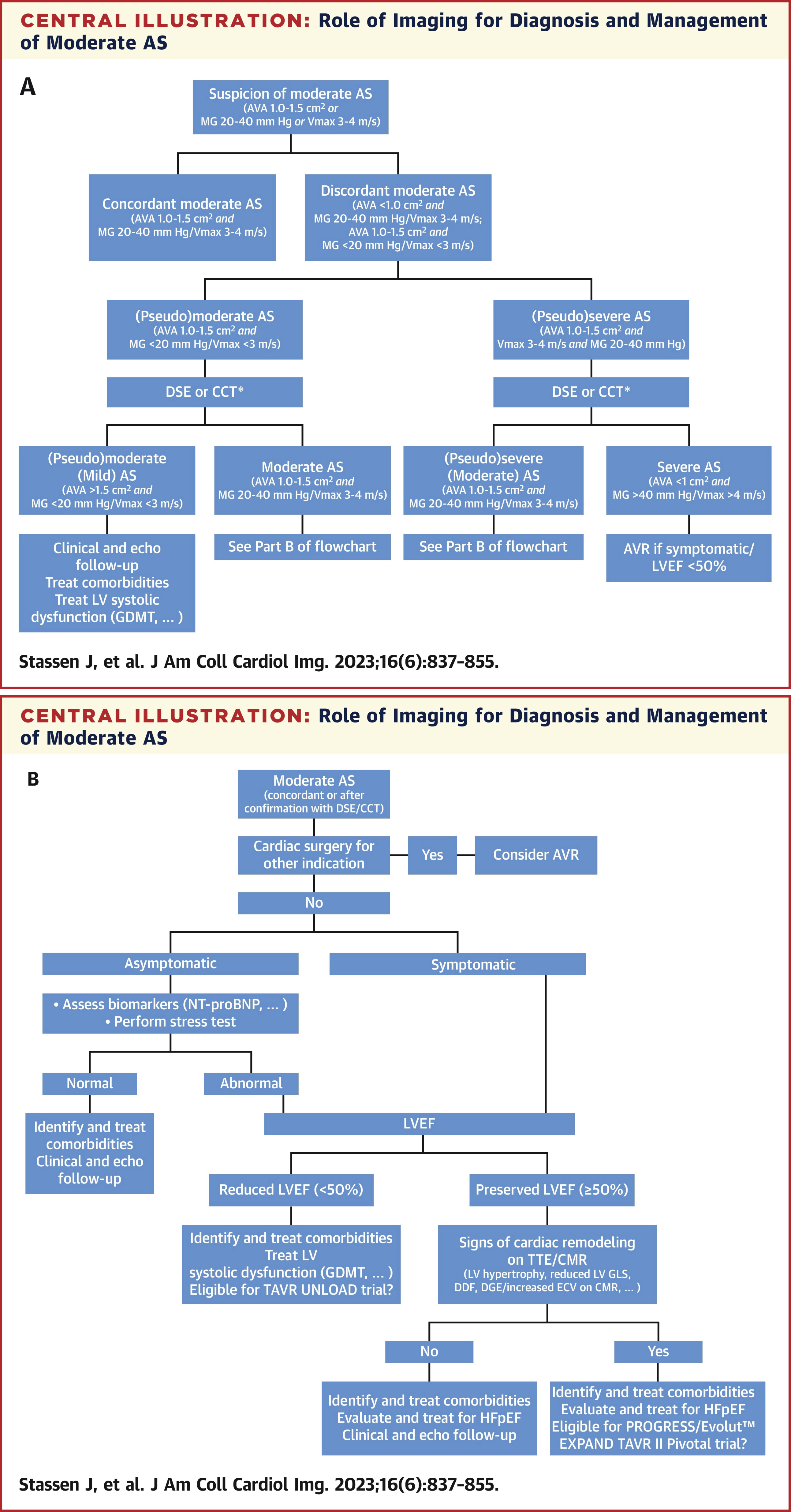
Patients with moderate AS constitute a heterogeneous population that includes asymptomatic versus symptomatic patients, less severe AS (e.g., AVA 1.5 cm 2 ) versus more severe AS (e.g., 1.1 cm 2 ), and few versus multiple comorbidities. The current treatment of patients with moderate AS should first focus on the recognition and appropriate management of comorbidities and left ventricular systolic and diastolic dysfunction (Figure 3).
 © 2023 Elsevier. Reprinted with permission from *JACC: Cardiovascular Imaging*, Volume 16, Issue 6, June 2023, Pages 837-855. Available at: http://www.sciencedirect.com/science/journal/1936878X
© 2023 Elsevier. Reprinted with permission from *JACC: Cardiovascular Imaging*, Volume 16, Issue 6, June 2023, Pages 837-855. Available at: http://www.sciencedirect.com/science/journal/1936878X
Figure 3: Role of imaging for diagnosis and management of moderate AS.
View Figure 3
Currently, there is no medical treatment for moderate AS. Recent data from the ASTRONOMER trial (Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin) showed that patients with preexisting mild to moderate AS and elevated levels of lipoprotein (a) and oxidized phospholipids had a faster rate of AS progression and a greater need for AVR. It has been demonstrated that PCSK9 inhibitors and mipomersen (recently approved) reduce lipoprotein (a) by 20% to 30%, and emerging RNA-targeted therapies have shown to reduce lipoprotein (a) by > 80%. These new therapies are expected to be evaluated for their potential to reduce or halt the progression of mild to moderate AS [10,27,29].
There are no published randomized trials evaluating the risk-benefit relationship of AVR in patients with non-severe AS. However, given the progressive nature of AS, current guidelines recommend that AVR should be considered for patients with moderate AS undergoing cardiac surgery for another indication (Class IIb indication in the 2020 American College of Cardiology/American Heart Association [ACC/AHA] guidelines and Class IIa indication in the 2021 European Society of Cardiology/European Association for Cardio-Thoracic Surgery [ESC/EACTS] guidelines).
Considering the increased risk of mortality, concomitant myocardial damage, and rapid progression, current evidence suggests that patients with moderate AS may benefit from early intervention, especially when AS-related symptoms are present along with signs of LV dysfunction [5].
In the international, retrospective, multicenter ATLAS TAVI registry, the outcomes of patients with severe or moderate AS with reduced LVEF undergoing percutaneous AVR versus medical therapy were investigated. The main findings were: 1) Among patients with AS and reduced LVEF, those undergoing percutaneous AVR were older, more symptomatic, and had more severe disease compared to those receiving medical therapy; 2) Percutaneous AVR was a strong predictor of overall and cardiovascular survival in patients with reduced LVEF and non-severe AS (moderate or pseudo-severe); 3) In patients with reduced LVEF and non-severe AS, those undergoing percutaneous AVR showed significantly lower 2-year rates of all-cause mortality and cardiovascular mortality compared to the cohort receiving medical therapy [30].
Another study derived from the TOPAS (True or Pseudo-Severe Aortic Stenosis) Registry showed benefits in patients with pseudo-severe AS undergoing early percutaneous AVR compared to the conservative strategy. Due to the findings in these studies, clinical focus should emphasize not only on the severity of AS but also on the complex interaction between LV myocardium and ventricle-valve afterload [8].
Currently, three prospective randomized controlled trials are underway, comparing the outcomes of patients with moderate AS undergoing percutaneous intervention versus conservative treatment: The TAVR UNLOAD (Edwards Lifesciences, NCT02661451), the PROGRESS Trial (Edwards Lifesciences, NCT04889872), and the EXPAND TAVR II Pivotal Trial (Medtronic, NCT05149755), which will clarify the current causal and prognostic role of valvular disease in these patients [5].
Screening for concomitant cardiovascular comorbidities in patients with paradoxical AS is recommended, as treating these comorbidities may improve symptoms or prognosis [8].
According to current guidelines, follow-up of patients with moderate AS should be every 1-2 years [6,7]. However, as demonstrated in the present studies in patients with moderate AS with different profiles according to their flow patterns, they may need to be followed more closely [4,8].
The authors have no conflicts of interest to disclose.