Background: Stanford A aortic dissection is a surgical emergency. Its misleading clinical presentations sometimes makes its diagnosis difficult.
Case presentation: We describe the case of a 58-year-old black male smoker patient with untreated hypertension admitted to the emergency room without symptoms, having presented a few hours earlier, moderate chest pain with transient sweating. The clinical examination found blood pressure asymmetry and a weaker radial pulse on the right arm. The ECG showed nonspecific abnormalities. D dimers were greater than 20,000 µg/L. Chest CT angiography showed Stanford type A aortic dissection. The patient underwent emergency surgery with aortic valve replacement, placement of a supracoronary tube and repair of the aortic pellet with simple postoperative course.
Conclusions: Although Stanford A aortic dissection is rare, clinician should be aware of it while facing a patient with transcient chest pain and cardiovascular risk factors, especially high blood pressure.
Aortic dissection, Blood pressure asymmetry, D dimers, Thoracic CT angiography
AD: Aortic Dissection; CT: Computed Tomography; ECG: Electrocardiogram; IRAD: International Registry of Aortic Dissections
Stanford A aortic dissection (AD) is a surgical emergency. In the absence of treatment, it could lead to the death of the patient within few hours [1]. Typically, diagnosis of AD is suspected in a patient presenting with intense, permanent and migrating chest pain. However, in less than 5% of cases, patients are painless [1]. This is the case for our patient. When he was admitted to the emergency room, he no longer had chest pain or any symptoms.
This is the case of a male patient, 58-years-old, black. He has untreated high blood pressure, an active tobacco consumption at 10 pack years. He had a normal coronary angiogram 13 years earlier prescribed for chest pain, as well as a drained post-traumatic right pneumothorax more than 10 years ago. He was taken to the emergency room by firefighters for chest pain. Indeed, he would have presented for a few hours before the consultation, chest pain of moderate intensity, mediothoracic, non-migratory, associated with sweating and a feeling of fatigue. These symptoms occured when driving a motorcycle. Note that the chest pain disappeared spontaneously without taking medication. Faced with these symptoms, the patient was transported to the emergency room, where upon admission he no longer presented any symptoms. On clinical examination, he was conscious and cooperative, he had blood pressure asymmetry with 152/59 mmHg in the right arm and 131/60 mmHg in the left arm, i.e. a difference in systolic blood pressure of more than 20 mmHg, a radial pulse at 73 beats per minute regular on both arms, but with lower volume on the right arm. The femoral pulses were symmetrical. Cardiac auscultation was normal. The patient showed no signs of heart failure, in particular, no jugular venous turgor, no hepato-jugular reflux, no edema of the lower limbs. The patient was eupneic, pulmonary auscultation was normal, respiratory rate was 18 cycles per minute. There was no sign of deep vein thrombosis of the lower limbs. The abdomen was supple, not painful. Furthermore, the patient had no particular complaint.
The biological parameters were as follows: Hemoglobin: 134 g/L, platelets: 153 × 10 9 /L, leukocytes: 12.0 × 10 9 /L, urea 7.0 mmol/l, creatinine 108 µmol/l, GFR CKD-EPI 64 ml/min , Na 138 mmol/L, K 3.5 mmol/L, Cl 103 mmol/L, glucose 6.7 mmol/L, creatine kinase 97 IU/L, D dimers > 20000 µg/L, TP 67%, INR 1.3, TCA patient/control ratio 1.22, CRP 1.1 mg/L, ALT 27 IU/L, AST 33 IU/L.
The electrocardiographic tracing showed a sinus rhythm at 79 beats per minute, electrical left ventricular hypertrophy with a Sokolov index of 38 mm, a deviated axis to the left, negative T waves in the inferolateral derivations. Figure 1 below shows the electrocardiogram (ECG).
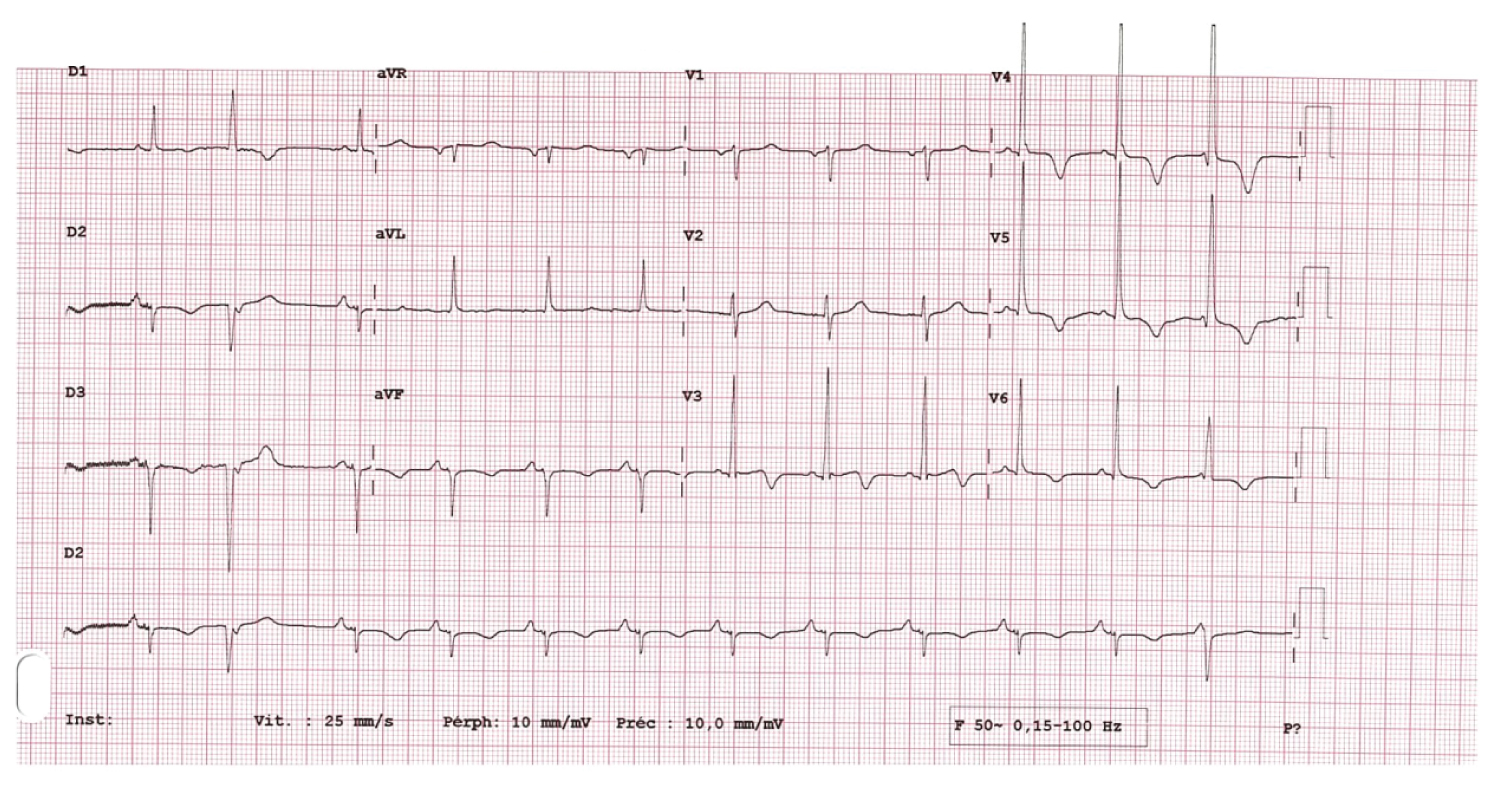 Figure 1: Electrocardiogram.
View Figure 1
Figure 1: Electrocardiogram.
View Figure 1
On the aortic thoracic computed tomography (CT) angiography, we found a type A aortic dissection involving the brachiocephalic arterial trunk, the right subclavian artery, the right carotid artery. This dissection extends along the aorta to the right iliac artery. The right and left coronary arteries, the two renal arteries, the superior mesenteric artery, arising from the true channel, the nascent celiac trunk straddling the true and the false channel. The inferior mesenteric artery arising from the false channel. There was no pulmonary embolism. Images A and B of Figure 2 below represent respectively a transverse section and a longitudinal section of the thoracic computed tomography (CT) angiography. The arrow indicates the false aortic channel.
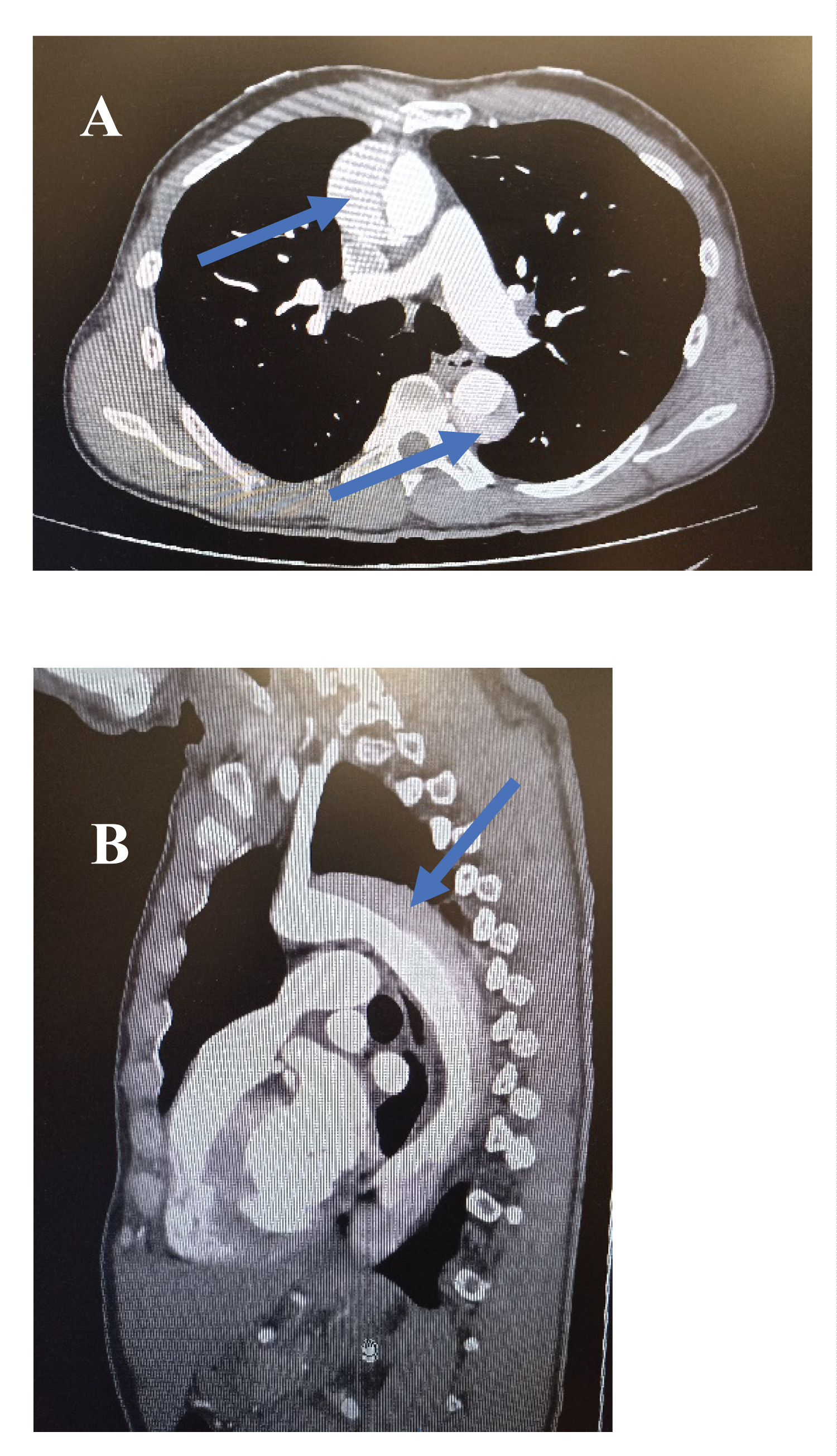 Figure 2: Computed tomography (CT) sections.
View Figure 2
Figure 2: Computed tomography (CT) sections.
View Figure 2
After conditioning with a multiparameter scope, placement of a venous line, the patient was taken urgently by medical transfer to the cardiac and vascular surgery department of a nearby hospital where he underwent aortic valve replacement, placement of a supracoronary tube with repair of the aortic pellet. The postoperative course was simple. The patient went back home with a treatment including: acetyl salicylic acid, bisoprolol, ramipril, urapidil, nicardipine. A follow-up appointment one month postoperatively with control thoracic angioscan was reassuring. He will be seen again six months later.
We report a case of Stanford type A AD of an asymptomatic patient on arrival at the emergency department. According to data from the International Registry of Aortic Dissections (IRAD), AD concerns the male subject in 2/3 of cases. It affects patients aged 63 years on average and in more than 60% of cases, it is type A AD. Arterial hypertension is a common comorbidity found in these patients with aortic dissection, especially more if it is not controlled [1]. The demographic characteristics of our patient were fully consistent with the literature.
More and more cases of non-painful or minimally painful AD are described in the literature [2-4]. Sometimes they present with atypical symptoms such as syncope, seizures, paraplegia, vertigo, atrial fibrillation, ileus [4-6]. These varied symptoms may reveal ischemic organ damage related to aortic dissection. Apart from arterial hypertension, several comorbidities have been found in patients with AD. These are diabetes mellitus, a history of AD, Marfan's disease, atherosclerosis, a history of heart surgery [1]. It is therefore advisable to systematically look for them, because in a non-painful patient, they could make it possible to arouse the clinical suspicion of an AD.
The pain in the AD is secondary to the stretching of the aortic adventitia caused by the dissecting hematoma. The cessation of the dissecting process, or the presence of one or more reentry gaps allowing the tensioning of the adventitia to be reduced, could perhaps explain the spontaneous cessation of pain in this patient. This rare mode of presentation of chest pain during AD is falsely reassuring. Rapid periods of acceleration and/or deceleration are reported as circumstances in which AD occurs [7,8]. Thus, the fact that this patient was a motorcycle driver when the initial chest pain occurred is of paramount clinical importance.
In a retrospective series of 159 cases, Hirata, et al. found that only 27% of patients with type A AD have a normal ECG. The most significant abnormalities being ST segment depression and T wave abnormalities, observed in 34% of cases [9]. The latter would be associated with a high incidence of shock and pericardial tamponade not observed in our patient, although he presented with inferolateral T wave inversion. The latter would certainly be related to left ventricular hypertrophy, the electrical signs of which were found on the electrocardiogram. During AD, levels of D dimers are elevated. They are higher in type A AD and increase over time. Thus, in a group of patients with type A AD, at the 6th hour of admission, the mean D dimer values were 719.5 ± 184.2 µg/L [10]. This value is significantly lower than that of our patient at the second hour after admission, indicating a high risk of clinical deterioration.
We can retain from this clinical case that in order not to misunderstand AD requiring emergency surgery, it is appropriate for the clinician in the emergency department, faced with a patient with cardiovascular risk factors and having presented chest pain, to look for a blood pressure and pulse asymmetry, D-dimer assay and, if indicated, aortic chest CT angiography.
The authors would like to thank and acknowledge the patient and his family.
GK: Involved in data collection, writing, and preparing the original draft. JG: Involved in surgical operation, clinical care of the patient and reviewing the article. YL, KB: involved in the clinical care of the patient, reviewing the article. AG, AC: involved in reviewing the article. All authors read and approved final manuscript.
None.
Data will be available on request.
Authors declare no conflict of interest.