Human sexual development starts early in fetal life and is completed in adulthood when the gonads have developed to their full reproductive potential. The HPG axis is a highly complex unit that influences fertility success in men and women [1]. The development, structure, and function of the HPG axis between males and females will be compared and differentiated below (Table 1) [2-8].
Table 1: Comparison of HPG axis of Males and Females. View Table 1
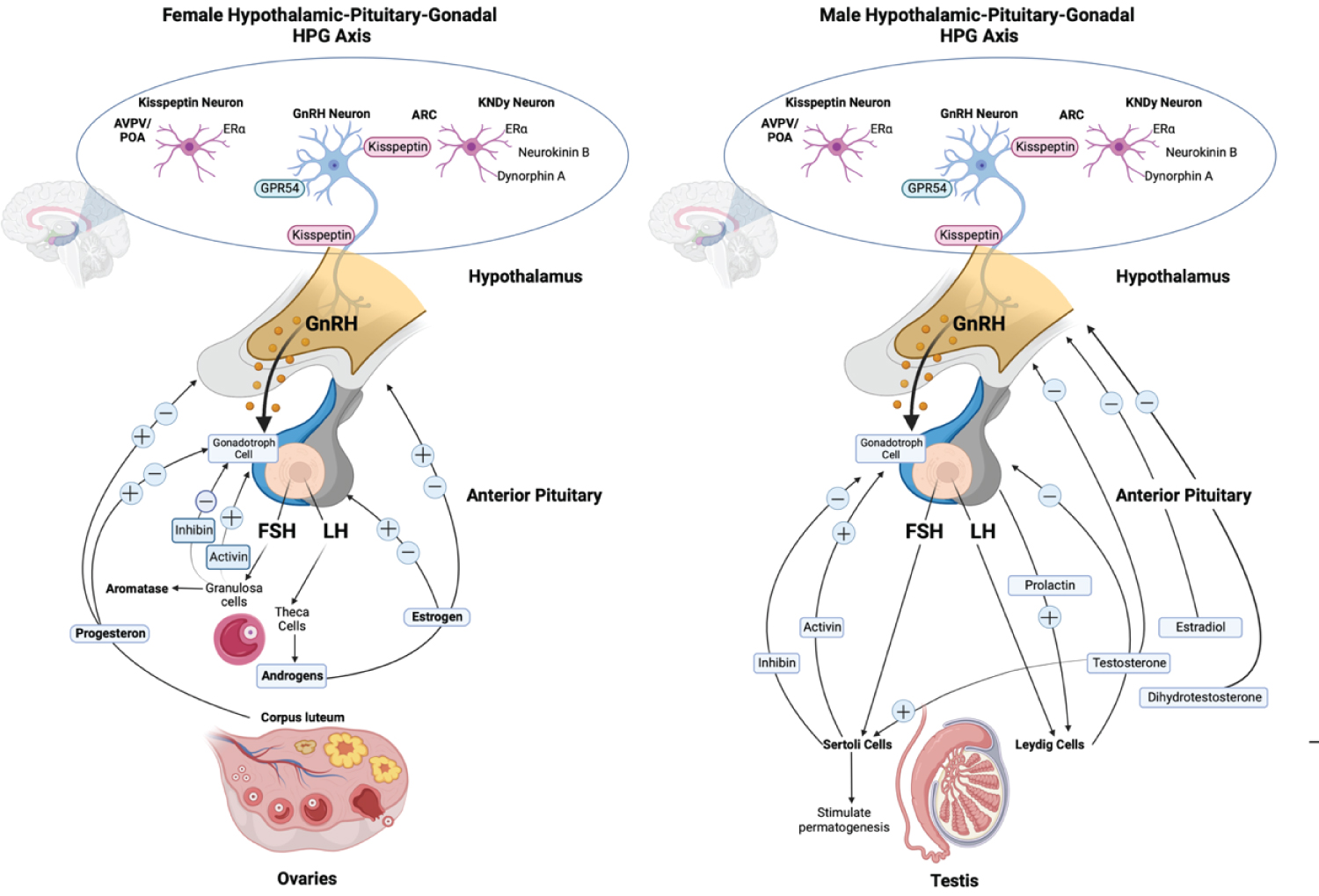
In both sexes, the physiological link of the HPG axis initiated when the hypothalamic peptide (GnRH) released into the anterior pituitary, triggers the production of gonadotrophins (LH and FSH), then stimulates spermatogenesis in males or oogenesis in females by binding to the gonad’s receptors which then release estrogen, progesterone, testosterone, and inhibins [1,4]. Complex feedback loops control the HPG axis’s principal hormone production process in males and females are shown in Figure 1 [2,3,9-13].
 (Image created with Biorender.com, adapted from Ozawa 2021)
(Image created with Biorender.com, adapted from Ozawa 2021)
Figure 1: Schematic of the different hormone production pathways of male and female Hypothalamic-pituitary-gonadal (HPG) axis. Kiss 1 neurons in ARC offer tonic stimulatory input to GnRH neurons which are adversely controlled by sex steroids. AVPV sex steroids activate Kiss 1 neurons. Trans-synaptic and glial inputs trigger GnRH release in the hypophysial bloodstream. GnRH modulates LH and FSH, which stimulate ovarian and testicular development. LH activates theca cells in females, which generate estrogen, primarily estradiol. FSH act on granulosa cells that modulate the aromatase. In the ovary, the corpus luteum release progesterone, which, together with estrogen, provides positive and negative feedback to the pituitary and hypothalamus. LH stimulates testosterone production in Leydig cells in males, negatively affecting the hypothalamus and pituitary. Testosterone is metabolized further to dihydrotestosterone and estradiol, negatively affecting the hypothalamus and pituitary. While FSH act on Sertoli cells that stimulate spermatogenesis [2,3,9-13].
View Figure 1
Regarding similarity, male and female hormone productions were initially influenced by the tonic release of kisspeptin by the arcuate and anteroventral periventral kisspeptin neuron, along with neurokinin B and dynorphin [9]. However, we believe there must be a gap of knowledge in terms of Kisspeptin production in males and females since the GnRH surge mechanism only exists in females.
The fundamental difference between both sexes was that males have very few Kisspeptin neurons, while females have many Kisspeptin neurons in the AVPV [2]. It also reflects the morphological differences, such as the amount of cell bodies and fiber density that influence higher input to GnRH neurons in females. In females, the presence of sex hormones stimulates Kiss1 expression as the cellular basis for estrogen’s positive feedback stimulation of the preovulatory GnRH surge [2].We need to better understand the influence of Kisspeptin in the switching mechanism affected by the steroid hormones from negative to positive feedback, which does not exist in males, not only differentiating Kisspeptin morphologically (Table 1).
In males and females, GnRH neurons are directly activated by kisspeptin, produced in the hypothalamus and thought to affect GnRH activity differently. The Kisspeptin 1 gene strongly stimulates GnRH secretion [14]. But the amount of Kisspeptin differs between men and women is unclear. Both sexes have the mechanism of GnRH pulsatility, which is thought to be regulated by ARC and AVPV nuclei [15].
In females, GnRH surge is known to be LH triggered, which will cause the LH surge. In males, the GnRH-LH surge may not occur due to the absence of estrogen and estrogen-sensitive cells [2,16]. Other studies found Kisspeptin cell bodies in the AVPV act as LH surge generator, while KNDy neurons in the ARC as pulse generators [3]. Different neurons and regions of kisspeptin stimulus will affect the GnRH function. Hence, the LH surge activity only happens in females (Table 1).
In females, The AVPV is the focus of positive feedback from estrogen while the ARC is the strong target of estrogen’s negative feedback [17-19]. Estrogen's stimulatory effects on the surge mechanism are assumed to be mediated by Era which expressed by nearly all Kiss1 neurons, and only females have significant amounts of Kiss1 cells [2]. Estrogen can stimulate ovulation and LH secretion in females [2,20].
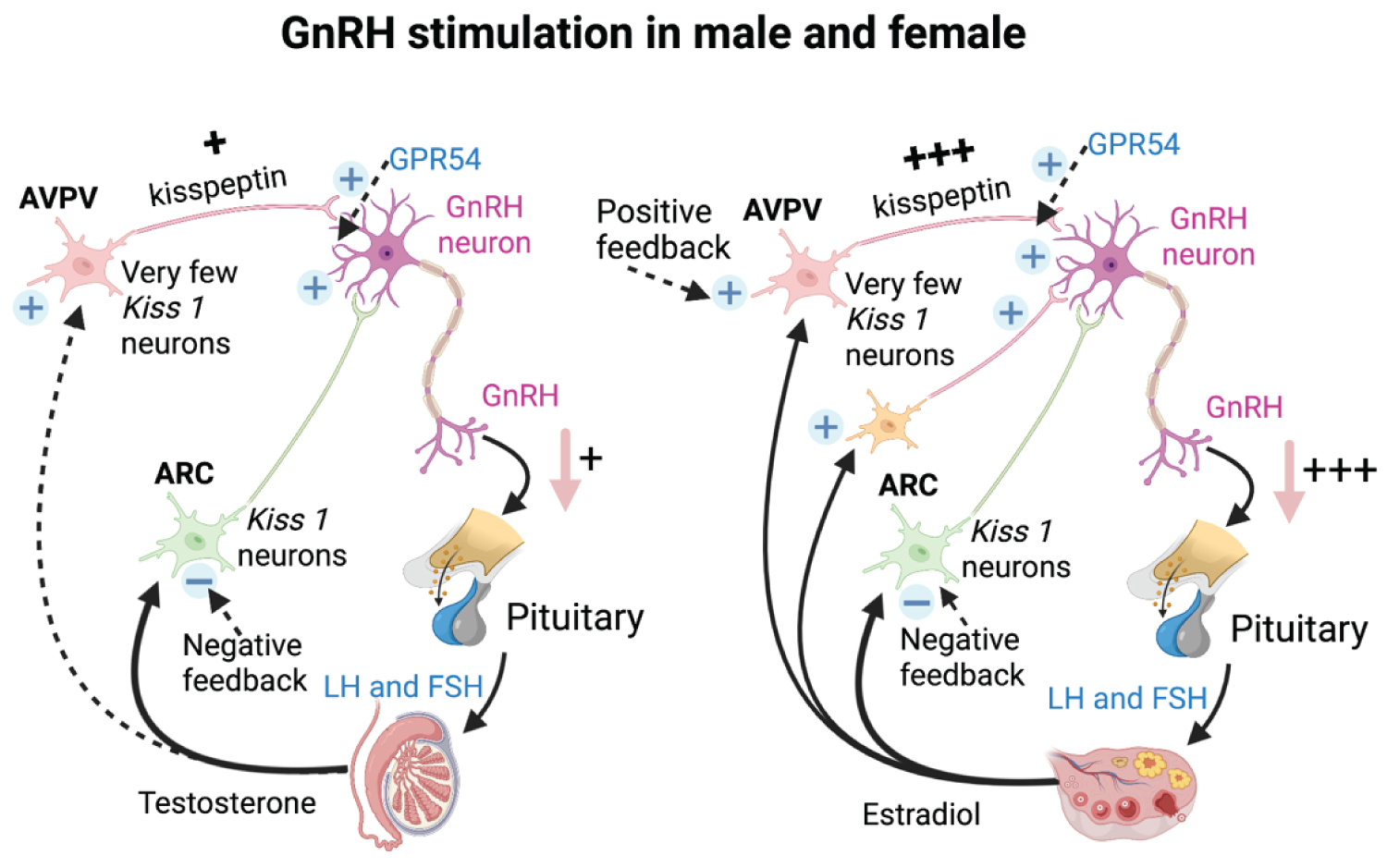
Kiss 1 cells are more prominent in females than in males [16,21]. Kisspeptin fibers in the AVPV of sexually mature females are 15-fold higher in mice than in rats [22,23] (Figure 2). It was found by the amount and distribution of immunolabelled kisspeptin in the infundibulum, which is higher in women [24].
 (Image created with Biorender.com, adapted from Kauffman, 2007)
(Image created with Biorender.com, adapted from Kauffman, 2007)
Figure 2: The schematic comparison of the Kisspeptin pathway in GnRH stimulation of male and female. Kisspeptin from Kiss 1 neurons induce GnRH secretion. The amount of kisspeptin generated differently is higher in female than male along with the sex hormones regulations [2,15].
View Figure 2
Kisspeptin from Kiss 1 neurons induce GnRH secretion. The amount of kisspeptin generated differently is higher in female than male along with the sex hormones regulations.
The pituitary or hypophysis gland is crucial in neuroendocrine control, comprised of various cell types that release hormones in blood circulation in both sexes [25]. The pituitary gland is located underneath the hypothalamus and is divided into adenohypophysis and neurohypophysis. Gonadotrophs are pituitary gland cells with GnRH receptors and generate FSH and LH in males and females.
In both sexes, LH and FSH are made up of general α and specific β subunits essential for the heterodimer’s biological activity. The variable size and electron density of gonadotrophic secretory granules distinguish them. The granule population in males rat gonadotroph is unique, while females rat gonadotroph has less diversity in secretory granules [26].
The size of the hypophysis gland is more extensive in females than in males. The hypophysis reaches the maximum size under hormonally active situations such as pregnancy and puberty. Study shows that the median height of the hypophysis gland under a year and the 18-year-old group was 3.81 ± 0.68 and 8.48 ± 1.08 mm for girls and boys was 3.91 ± 0.75 and 6.19 ± 0.88 mm. In terms of size, this might occur due to LH hypersecretion [27-29].
The FSH and LH affect both ovaries and testes differently regarding steroid and gametes production. In terms of gametogenesis, FSH plays an essential role in both sexes. In females, FSH promotes follicular maturation and granulosa cell estrogen synthesis. In steroidogenesis, FSH attaches to FSH receptors on granulosa cells causing androgens production in internal theca cells to aromatize estrogens [25]. In contrast with males, after FSH stimulates the synthesis of androgen receptors, inhibin production and calcium signaling on FSH receptors will stimulate spermatogenesis on Sertoli cells [6] (Figure 1).
LH has an essential role in steroidogenesis. In females, LH promotes theca cell androgen production to cause ovulation and maintains corpus luteum progesterone production [26]. On internal theca cells, LH binds in the early follicular phase to LH-receptor surface, increasing androgen synthesis (androstenedione, testosterone) and follicle development [6]. In males, LH binds exclusively to Leydig cells (LH receptors) to stimulate testosterone synthesis, whereas prolactin and inhibin may function simultaneously to boost LH production.
As explained earlier, GnRH/LH surge marks the difference between both sexes. In females, LH pulses change every 90-100 minutes to every 4-8 hours from the early follicular phase to the late luteal phase [6]. While in males, LH pulses occur every 55 minutes in mature males, testosterone is the primary driving factor that governs LH synthesis, either directly or indirectly, via aromatization to estradiol [6] (Figure 3 and Figure 4) [30-32].
 (Image created with Biorender.com, adapted from Duraijayanagam, 2015)
(Image created with Biorender.com, adapted from Duraijayanagam, 2015)
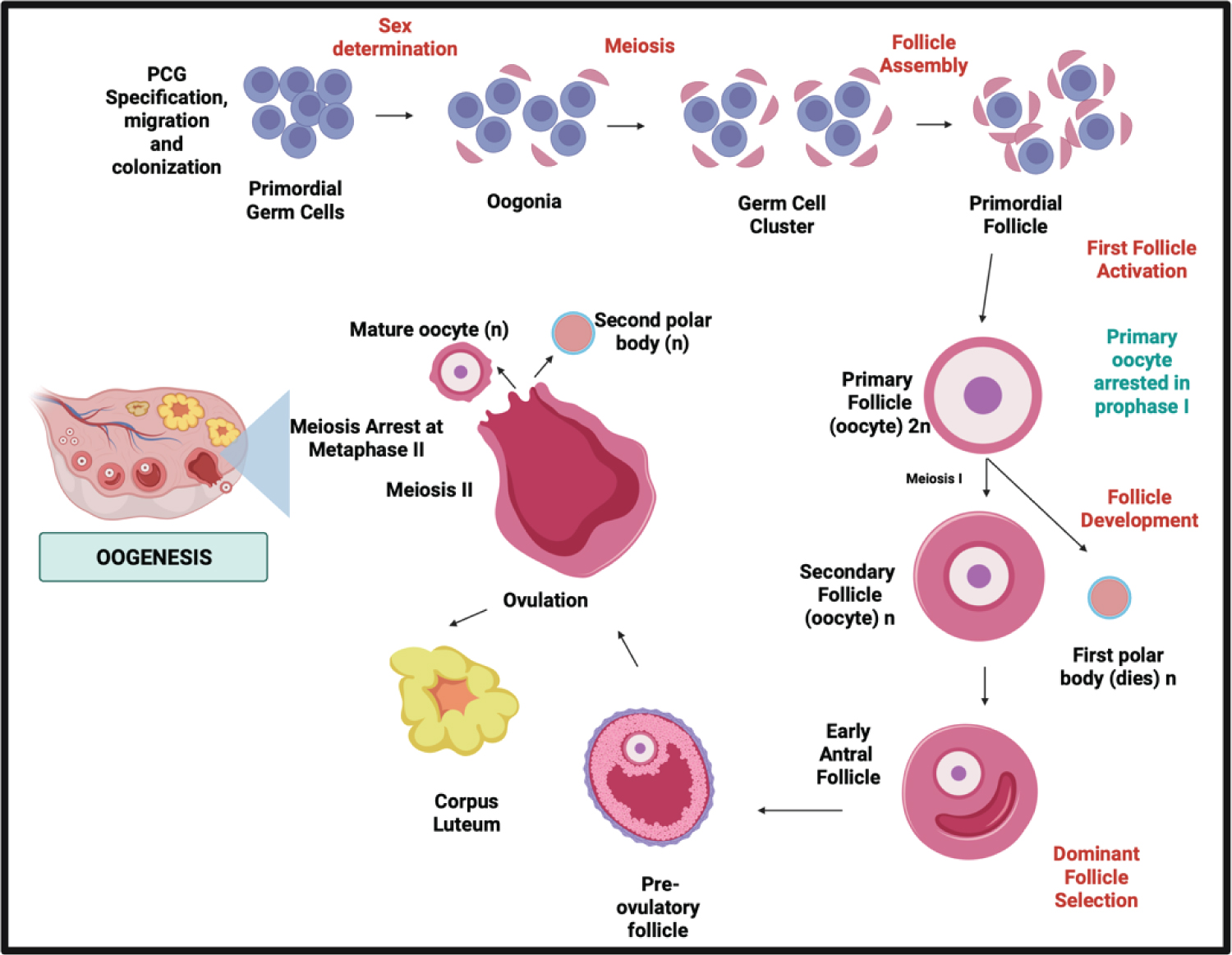
Figure 3: The schematic comparison of meiotic phases in Spermatogenesis in males and Oogenesis in females.
In this Oogenesis phase, the periodical process of oocyte production through females life and the meiosis stages of oocytes also the sex steroid produced has been the complete difference to the spermatogenesis process [30,31].
View Figure 3
 (Image created with Biorender.com, adapted from Cordeiro, 2015)
(Image created with Biorender.com, adapted from Cordeiro, 2015)
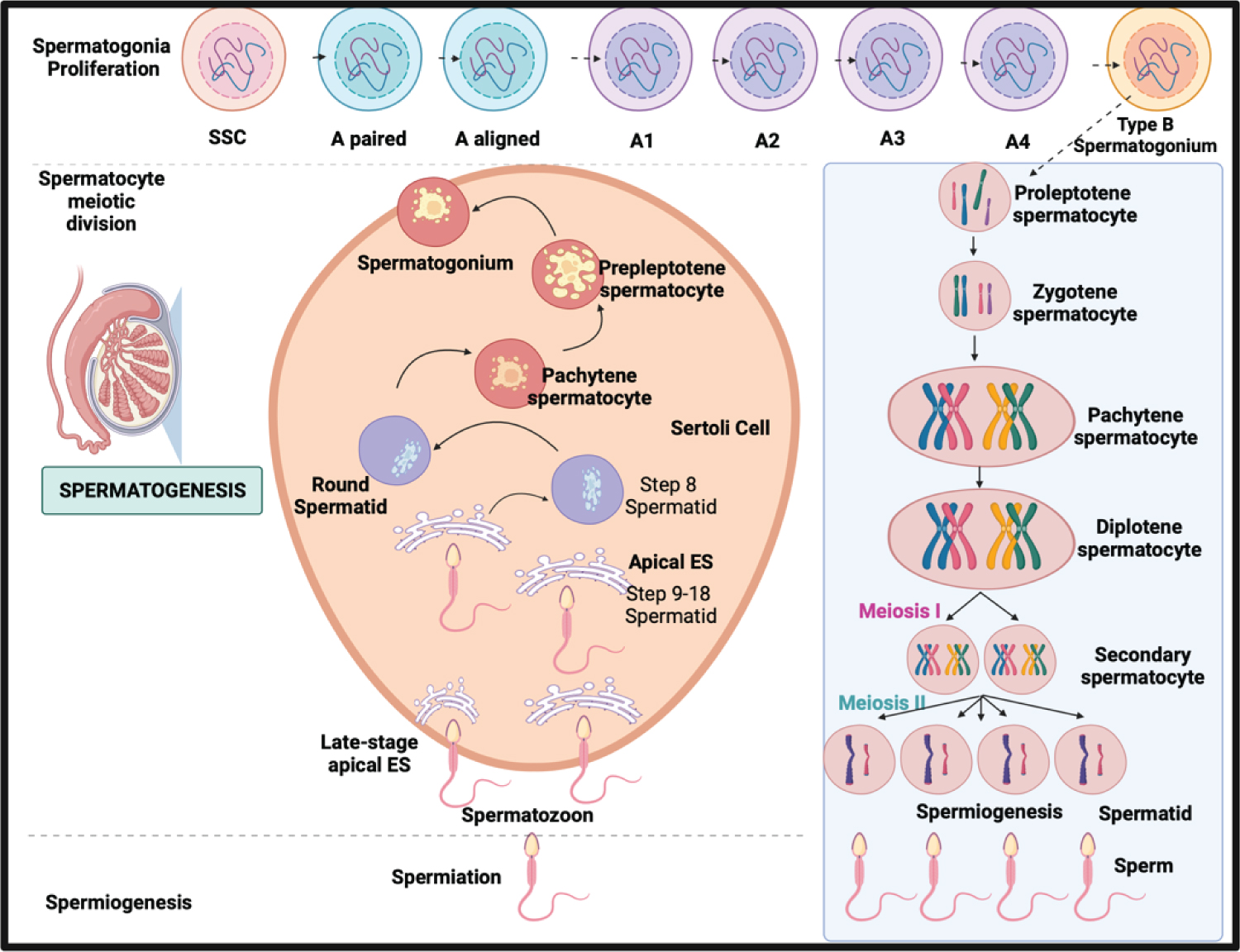
Figure 4: The schematic comparison of meiotic phases in Spermatogenesis in males and Oogenesis in females.
In this Spermatogenesis phase, the continuous process of sperm production through the male's life and the meiotic process of the gametes distinguished spermatogenesis from oogenesis [30-32].
View Figure 4
The meiotic phase between both processes distinguishes oogenesis from spermatogenesis in males and females. In males, the process is continuous without following episodic hormonal phases. In females, in the dictyate stage of meiosis I until LH promotes ultimate oocyte maturation which is periodically according to the hormonal phases [5]. In males, spermatogenesis takes 74 days in the testicular seminiferous tubules to complete SSC differentiation: mitosis, meiosis, and spermiogenesis [7], while In females, it takes 28-35 days for a cycle through different phases, and only one dominant follicle is produced [33,34].
In spermatogenesis, spermatogonia are undifferentiated, capable of self-renewal and differentiation into A-paired (Ap) spermatogonia. A-aligned (Aal) spermatogonia develop through sequential mitosis. Sertoli cell-derived, FSH-induced growth factors [7]. While in female oogenesis, the mitotic process occur before birth and undergoes an arrest in prophase I. In contrast to males, which mitotic divisions change into A2, A3, A4, and B spermatogonia [33].
Spermiogenesis takes 26 days, as secondary spermatocytes go through meiosis II when sister chromatids split to form haploid round spermatids. While in females, after puberty, oocyte meiotic maturation begins As a result of the LH surge, the oocyte progresses to metaphase II, culminating in the ejection of the first polar body [34]. Meiosis restarts after the LH surge when cumulus cells expand, mediated by the formation of hyaluronic acid by the cumulus cells. Upon an LH trigger, Nppc mRNA levels (encoding CNP) decline, resulting in reduced synthesis and diffusion [34]. Finally, developed spermatozoa are highly specialized cells that need active mobility to enter the female genital canal and reach the oocyte [8]. In females, the oocyte will complete meiosis if it is fertilized.
Overall, the similarity and differences between both sexes are essential in reproduction. In males and females, the similarity was reflected in the hypothalamic-pituitary structure, hormonal feedback, and GnRH pulsatility. The differences between both sexes were reflected in kisspeptin, gonads (ovary and testes), sex steroids (estrogen and testosterone), number of gametes produced (oocyte and sperms), and duration (periodical and continuous process) see (Table 1).