Olfactory groove meningiomas (OGMs) are rare intracranial tumours that arise from the anterior cranial fossa, typically originating at the cribriform plate, planum sphenoidale, or fronto sphenoidal suture. Due to their slow-growing and insidious nature, OGMs often go undetected until they reach considerable size, making them among the largest central nervous system (CNS) tumours at diagnosis. Common presenting symptoms include headache, anosmia, personality changes, and visual impairment; however, these can be subtle and easily overlooked.
We present the case of a female in her early 70s who presented with an isolated 5-week history of progressive visual deterioration, initially affecting the right eye. Notably, she exhibited no other common neurological symptoms such as headache, seizures, or personality change. Imaging via CT and MRI revealed a giant olfactory groove meningioma measuring over 6 cm, with mass effect, midline shift, and encasement of both internal carotid arteries.
The patient underwent a bifrontal craniotomy and subtotal tumour resection, achieving approximately 80-90% removal. Critical neurovascular structures, including the right optic nerve and anterior cerebral arteries (ACA), were successfully preserved. Histopathological analysis confirmed a WHO Grade II atypical meningioma with brain invasion but a low Ki-67 proliferation index. Given the incomplete resection and tumour grade, adjuvant postoperative radiotherapy was recommended to reduce recurrence risk.
The patient’s post-operative course was favourable, with notable improvement in visual acuity and field over the following weeks. This case underscores the stealthy and potentially dangerous growth of OGMs, particularly when classical symptoms are absent. It highlights the importance of early imaging in unexplained visual loss and the role of a multidisciplinary approach in managing complex skull base tumours. Greater awareness of atypical presentations may aid in earlier diagnosis and improved outcomes.
Visual loss, Olfactory groove meningioma, Atypical presentation, Craniotomy, Case report
OGM: Olfactory Groove Meningioma; CNS: Central Nervous System; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; ICA: Internal Carotid Artery; ACA: Anterior Cerebral Artery; WHO: World Health Organization; BMI: Body Mass Index; RE: Right Eye; LE: Left Eye; GCS: Glasgow Coma Scale; SOL: Space Occupying Lesion; CUSA: Cavitronic Ultrasonic Surgical Aspirator; MCA : Middle Cerebral Artery; HPF: High-Power Field; ACoA: Anterior Communicating Artery; RT: Radiation Therapy; SST2A: Somatostatin Receptor 2A; H&E: Haematoxylin and Eosin
Meningiomas are the most common primary tumour of the CNS. They originate from the arachnoid cells located on the inner surface of the dura. Most meningiomas are benign and frequently asymptomatic and hence are usually discovered incidentally on brain imaging. They have an incidence of 7.86 cases per 100,000 people per year [1]. The WHO classification published in 2016 categorises meningiomas into grades I-III, each requiring different treatment, including surgery and possible radiotherapy depending on the grade.
Olfactory groove meningiomas (OGM), originate in the anterior cranial fossa, most commonly at sites including the cribriform, planumsphenoidale, and the frontosphenoidale suture [2]. They are slow-growing tumours, often growing bilaterally but asymmetrically. They have an incidence of approximately 8-13% of all meningiomas [3].
We present a case of a large OGM causing only visual failure.
A 72-year-old female had a 5-week history of progressively worsening blurry vision with low mood. She then presented with right-eye (RE) visual loss. She had no headaches, vomiting, seizures or gait issues. Her past medical history includes hypertension, bradycardia, hypercholesterolemia, depression and an increased BMI. Her medication pre-admission to hospital included Losartan/Hydrochlorothiazide, Bisoprolol, Doxasosin, Amlodipine, Rosuvastatin, and Sertraline. Since admission, she was started on Esomeprazole, Dexamethasone, and Levetiracetam. A cranial nerve exam showed reduced visual acuity in her RE in all fields. The patient could perceive light but was unable to distinguish any objects. Other than impaired vision, neurological examination was unremarkable with a Glasgow Coma Scale (GCS) of 15/15.
An octopus visual field test was which showed the patient could not centrally fixate on the target in her RE. In her left eye (LE) she was unable to see centrally. However, a formal visual field test could not be conducted due to the patient’s visual failure.
Then scans including, CT Brain with and without contrast and MRI Brain with and without contrast were all conducted. The CT scans showed a very large planum sphenoidal and olfactory groove space occupying lesion (SOL) measuring approximately 6.2 cm × 5.5 cm × 3.4 cm in maximal dimension. Dorsally the SOL extended into the Sella and cavernous sinuses and overlaid the wing of the sphenoid on the left. Extensive vasogenic oedema in both anterior frontal lobes and mild midline shift to the right was also appreciated.
The SOL resulted in sulcal effacement and dorsal displacement of the corpus callosum and both lateral ventricles. Both supraclinoid segments of the internal carotid artery (ICA) were encased. There was no convincing hydrocephalus or haemorrhage.
MRI further validated the findings as well as suggested the tumour may extend through the cribriform plates which were difficult to appreciate on CT.
The radiological assessment concluded a Planum Sphenoidale and Olfactory Groove Meningioma was the most compatible diagnosis.
A couple of days after the scans the patient underwent a craniotomy. Cefuroxime was given as a prophylaxis and Losartan/Hydrochlorothiazide was held prior to surgery. Planning included a repeat CT Brain with Contrast preoperatively and using navigation stealth during operation. A bi-coronal incision was made and a bifrontal craniotomy followed. The dura matter was opened from lateral to medial and the meningioma was identified. An interhemispheric approach was used to debulk the tumour with a cavitronic ultrasonic surgical aspirator (CUSA). The middle cerebral artery (MCA) bilaterally, ACA and right optic nerve were identified and spared. The dural attachment to the anterior cranial fossa was also coagulated.
Histologic examination showed a tumour composed of epithelioid cells, with round to ovoid nuclei and nuclear pseudo inclusions, forming lobules and occasional whorls. Areas with mildly increased cell density and pattern less growth were identified, and there was multifocal brain invasion, which was highlighted by synaptophys in immunohistochemical staining. Other features of atypical meningioma, such as prominent nucleoli, small change or necrosis were not identified. The Ki-67 proliferation index was low, estimated at 1-3%.
A post-operative MRI brain with contrast was conducted. This showed 80-90% resection of the large bilateral meningioma. There was some residual tumour remaining in the olfactory grooves and encasing the inferior aspect of the falx at the anterior margin of the resection cavity as well as additional residual in the suprasellar cistern. There was also a new 2.6 cm haematoma in the lateral left frontal lobe. Contusional changes in the surgical cavity and additional infarction in both anterior frontal lobes were also evident.
The patient was monitored in the subsequent week. Her power and coordination in all four limbs were normal. An octopus visual field was also performed showing an improvement in her visual acuity and visual field. Her visual acuity in her RE and LE was 6/36 and 6/9 respectively. This continued to steadily improve over the course of the week.
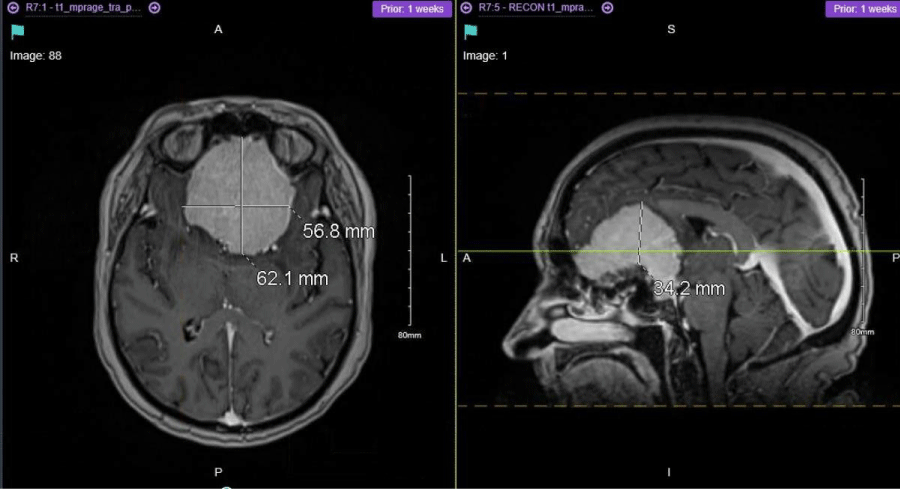 Figure 1: Preoperative MRI Brain showing a very large planum sphenoidal and olfactory groove space occupying lesion (SOL) measuring approximately 6.2 cm × 5.5 cm × 3.4 cm in maximal dimension.
View Figure 1
Figure 1: Preoperative MRI Brain showing a very large planum sphenoidal and olfactory groove space occupying lesion (SOL) measuring approximately 6.2 cm × 5.5 cm × 3.4 cm in maximal dimension.
View Figure 1
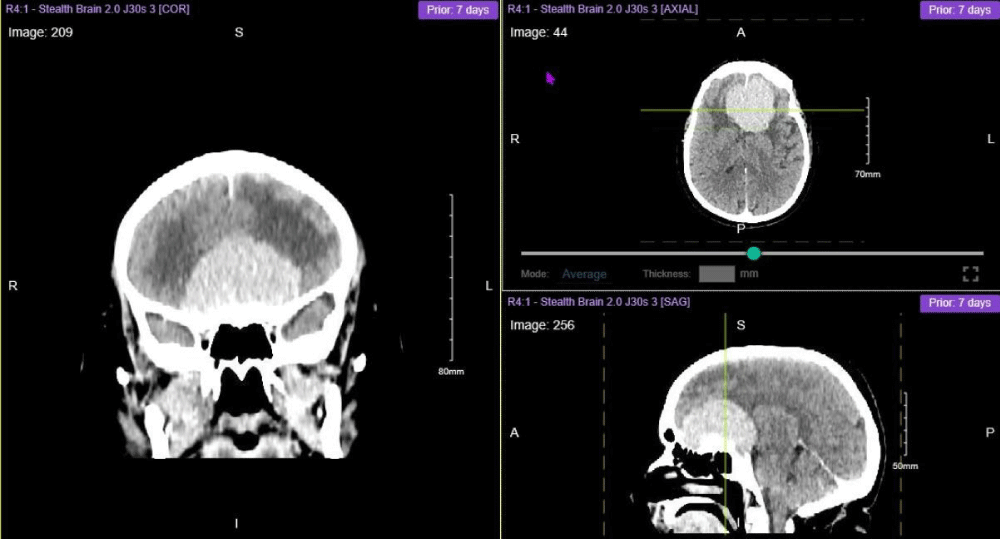 Figure 2: Preoperative CT Brain Stereotactic Planning with Contrast showing a very large planum sphenoidal and olfactory groove space occupying lesion (SOL) measuring approximately 6.2 cm × 5.5 cm × 3.4 cm in maximal dimension.
View Figure 2
Figure 2: Preoperative CT Brain Stereotactic Planning with Contrast showing a very large planum sphenoidal and olfactory groove space occupying lesion (SOL) measuring approximately 6.2 cm × 5.5 cm × 3.4 cm in maximal dimension.
View Figure 2
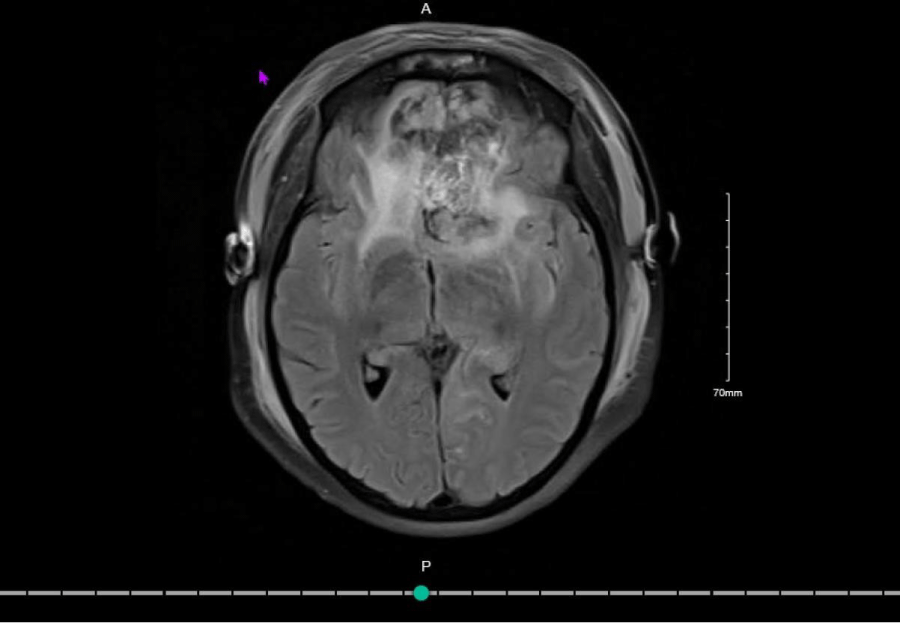 Figure 3: Postoperative MRI Brain which showed a 80-90% resection of the large bilateral meningioma.
View Figure 3
Figure 3: Postoperative MRI Brain which showed a 80-90% resection of the large bilateral meningioma.
View Figure 3
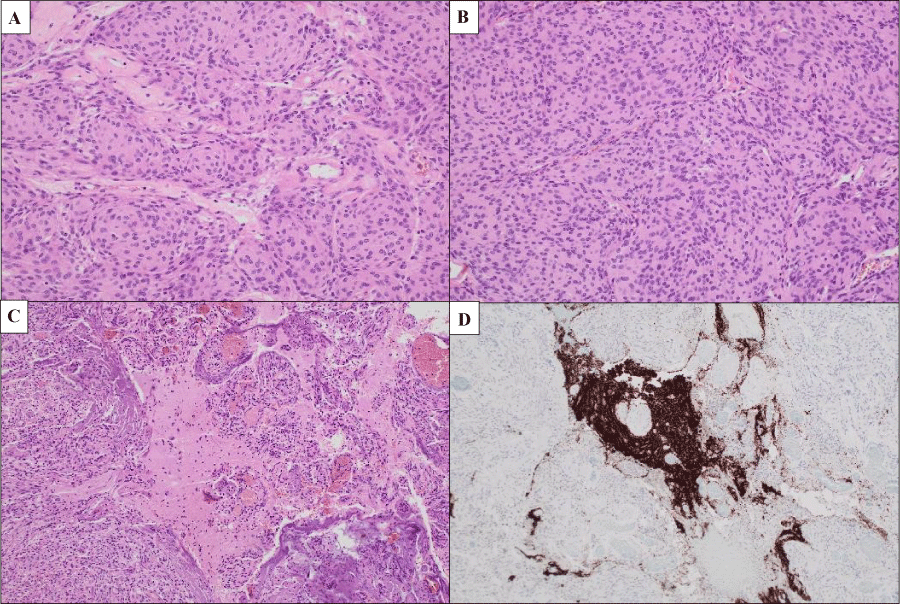 Figure 4: A) H & E-stained section showing epithelioid cells with a lobulated growth pattern forming occasional whorls (200 ×); B) Increased cell density with pattern less growth (sheeting) (200 ×); C) Brain invasion (100 ×), highlighted by synaptophys in immunohistochemical staining; D) (100 ×).
View Figure 4
Figure 4: A) H & E-stained section showing epithelioid cells with a lobulated growth pattern forming occasional whorls (200 ×); B) Increased cell density with pattern less growth (sheeting) (200 ×); C) Brain invasion (100 ×), highlighted by synaptophys in immunohistochemical staining; D) (100 ×).
View Figure 4
OGM scan present with a plethora of symptoms including headaches, nausea, and personality changes and if large enough, can lead to visual deficits [4]. Often these symptoms can be quite subtle and therefore underestimated which explains why OGMs can become one of the biggest intracranial tumours [5,6]. One possible explanation of why these symptoms can go under the radar is that the frontal lobes are among the brain regions with the greatest plasticity and resistance to displacement by anterior floor lesions [7]. The most common presenting symptom is headache which is apparent in up to 86% of cases and becomes increasingly more common as the tumour increases in size [8]. Anosmia and personality changes can also be seen in up to78% and 72% of cases respectively [8]. Visual impairment is the fourth most common symptom (24-61%).
Visual impairment occurs when the tumour exerts a downward pressure on the optic nerve and chiasm from above as it increases in size [6]. This could further progress to optic atrophy and present as Foster-Kennedy syndrome [6].
Interestingly in our case, the patient did not present with any of the most common symptoms except visual impairment. Additionally, she didn’t present with other less common symptoms such as seizures or intracranial hypertension. Our case highlights how common symptoms of OGMs may not present leading to the discrete and threatening growth of a giant tumour burden.
The insidious nature of OGMs highlights the importance of radiologic studies for diagnosis. The first reported radiologic diagnosis of a meningioma was in 1902 through radiography [9]. Nowadays, MRI is the standard modality of diagnosis, with CT scans also used in certain instances. OGMs can appear similar to other types of meningiomas however as seen in this case, they arise from the dura matter and overly the cribriform plate [9]. MRI reveals a dural- based, homogenously enhancing and well-circumcised lesion [10]. Information on the extension of the tumour and the dural implant is vital for surgical planning [7]. MRI also shows the relationship of the tumour to the optic nerve and chiasm as well as the neurovascular supply [9]. A recent review of 99 OGMs described anterior communicating artery (ACoA) involvement in 9% of cases [5]. CT scan is ordered on the condition that MRI suggests hyperostosis at the sight of implant [7]. As is our case, hyperostosis was susceptible on MRI and thus a CT scan was ordered to accurately pinpoint the hyperostosis throughout the planum sphenoidale.
Neuroimaging also plays a role in evaluating tumour size, including small (0-2 cm in diameter), medium (2-4 cm diameter) large (4-6 cm diameter), and giant (> 6 cm in diameter). The review of 99 OGMs described the mean tumour size as 5.4 cm and another study of 59 OGMs reported 18.7% of cases being giant [4,5].
In terms of treatment routine surveillance is an acceptable strategy however if the tumour is growing or causing symptomatology surgical resection is the gold standard [10]. Surgical approaches include bifrontal, pterional and fronto-orbito-basal [5]. Each approach isused in different circumstances and each possesses their own advantages and disadvantages. According to the literature the bifrontal approach allows for the best exposure of giant OGMs, the pterional approach is more commonly used for small OGMs and the fronto-orbito-basal approach had the highest tumour removal (Simpson I-III) with the lowest life threatening complications [5,6]. Surgery can result in improving or worsening patients vision. In a systematic review of 9 articles including 317 patients, 123 patients (39%) experienced visual improvement after surgery, 120 (38%) experienced visual stabilization, and 74 (23%) experienced visual worsening [11]. In this instance, the patient experienced an improvement in her vision.
Although radiologic appearance is often sufficient for diagnosing meningiomas, a histopathologic analysis is the only way to unequivocally confirm a diagnosis [10]. The WHO 2016 classification differentiates meningiomas into grades I-III [12]. Grade I meningiomas can present with a lot of different histological patterns including meningothelial and fibrous (which are the most frequent), transitional, psammomatous, angiomatous, microcystic, secretory, lymphoplasmacyte rich and metaplastic [12]. Grade I are the most common and have < 4 mitoses per 10 consecutive high-power fields (HPF) with no brain invasion [1]. Grade II or atypical meningiomas present with clear cell or choroid histology with amitoticrateof4-19 per 10 HPF or brain invasion [1,12]. If neither feature is present at least 3 of 5 of the histologic criteria must be evident. In our patient only 2 of the 5 histologic criteria were present but the diagnosis of Grade II atypical meningioma was made based on apparent brain invasion. As per Vranic A, et al. a Ki67 proliferation index over 4% is associated with an increased recurrence risk [13]. This is commonly used as complementary information rather than an indicator of tumour grade. Grade III or anaplastic meningiomas have papillary or rhabdoid histology or a mitotic rate of > 20 per 10 HPF [12]. Epithelial membrane antigen is the most used immunohistochemistry marker however recent studies show that somatostatin receptor 2A (SST2A) is a superior immunostaining target [10].
The degree of resection is measured using the Simps on grade, classified from 1-5 [1].
• Complete resection, with dural and bone resection
• Gross total resection with dural coagulation
• Macroscopic resection, without dural excision or coagulation
• Subtotal resection
• Biopsy
Rates of recurrence are impacted by the extent of resection, with the likelihood of recurrence substantially increasing in higher Simpson grades [10].
Our patient received a Simpson grade III resection. This was as much as safely could be done due to the encompassing of the ICAs with the A1 and A2 segments directly opposed to the tumour. Furthermore, both ACAs also appeared narrowed but patent.
If a meningioma is untreatable radiation therapy (RT) is a less effective but valid alternative [1]. More commonly, however, RT is used as an adjuvant treatment for decreasing the recurrence rate of meningiomas. This is only utilised in grade II and III tumours (although still controversial in grade II) with daily fractions over a 5-6 week period. The decision was made to send our patient for radiotherapy despite the Ki67 proliferation index being less than 4% because she underwent an incomplete resection.
This case report brings attention to the insidious nature of olfactory groove meningiomas. We wish to highlight how more common, less threatening symptoms may never present. This brings about the risk of extreme tumour growth and detection when a dangerous manifestation appears, such as vision loss.
We would like to express our sincere gratitude to the neurosurgery team at Beaumont Hospital for their invaluable support and clinical expertise in managing this case. Special thanks to the radiology and pathology departments for their contributions to diagnosis and analysis. We also acknowledge the patient and their family for their cooperation and consent in sharing this case for educational and research purposes.
The authors report there are no competing interests to declare.
No external funding was received for this case report.
All authors contributed equally to the conception, drafting, and final approval of this manuscript.
‘Everything has gone so well. I can’t believe how quickly my condition worsened. If you had told me at Christmas last year that I’d have brain cancer only months later I wouldn’t believe you. But everyone at the hospital has been great, the whole process was so smooth and I’m glad that my vision is starting to come back’.