The article aims to present partial results from an ongoing clinical trial, including fourteen patients undergoing endarterectomy with an innovative technique. The male gender accounted for 71.4% of the sample, with a mean age of 67.93 (± 7.75) years for the patients. Among comorbidities, systemic arterial hypertension was the most prevalent at 85.7%, followed by dyslipidemia at 57.1%. The mean carotid stenosis was 77.6% (± 7.82%). In 93.3% of the 15 procedures, videoangiography with the fluorescence module of the surgical microscope (Zeiss Pentero 800S®) showed arterial flow in the distal part of the internal carotid artery after the completion of endarterectomy, consistent with Doppler findings up to 24 hours. Therefore, videoangiography with fluorescein at the end of endarterectomy is a simple and innovative technique that, so far, demonstrates the safety of the procedure.
Carotid stenosis, Endarterectomy, Fluorescein
One of the most significant causes of ischemic stroke (IS) is carotid stenosis, accounting for over 20% of these events [1]. Low perioperative morbidity and mortality, along with a reduced incidence of recurrent stenosis, are crucial for the success of carotid endarterectomy (CEA). In this context, some authors advocate intraoperative monitoring to optimize the quality of endarterectomy, avoiding residual stenosis and luminal thrombus. Various methods have been described in the literature, such as Doppler and angiography [2]. Although cerebral angiography is considered the gold standard, it is invasive and not without complications. It has gradually been replaced by carotid Doppler, a non-invasive examination [2,3], although cases of cerebral ischemia have been reported even after satisfactory Doppler results [4].
In light of these considerations, angiographic markers coupled with light filters on surgical microscopes have proven to be effective and safe, as indicated by Haga, et al., in 2011, using indocyanine green to identify atheromatous plaques and provide final control of endarterectomy [5]. However, the financial cost of this marker may limit its use in the public health context. Partial results from the present study indicate that fluorescein, another type of tracer, represents an innovative and undocumented technique in the literature to assess carotid flow at the end of endarterectomy, being safe and less costly to the healthcare system.
It is an ongoing, non-randomized, and non-blinded clinical trial that started on March 1, 2021, following approval from the ethics and research committee of Santa Casa de Belo Horizonte, Minas Gerais, with the CAAE number 31701320.0.0000.5138. The primary objective is to demonstrate the safety of fluorescein in the intraoperative setting of carotid endarterectomy. The final study proposal aims to achieve a sample size of 50 patients.
The study design involves symptomatic and/or asymptomatic patients with carotid stenosis undergoing endarterectomy, with the intraoperative use of fluorescein coupled with the fluorescence module of the surgical microscope (Zeiss Pentero 800S ® ). All patients undergo preoperative Cervical Magnetic Resonance Angiography (MRA) as well as 3D Time-of-Flight Intracranial MRA (TOF). Follow-up includes scheduled appointments at 15, 30, 90, 180 days, and 1 year after endarterectomy, along with carotid Doppler ultrasound at 24 hours and 3 months post-procedure. Doppler results are interpreted based on the reference table suggested in the consensus of radiologists and ultrasonographers [6].
The inclusion criteria encompass symptomatic and/or asymptomatic patients with carotid stenosis between 70-99%, undergoing CEA with the use of fluorescein at the end of the entire procedure, and who agree to participate in the study by signing the Informed Consent Form. Exclusion criteria include patients for whom fluorescein was not used during CEA, those who are lost to follow-up for 1 year, or those who withdraw their consent to participate in the research.
The preoperative Cervical and Cerebral Vessels Magnetic Resonance Angiographies are performed using the Magnetic Resonance Imaging device (Philips Intera 1.5T ® ).
The 3D Time-of-Flight MRA of cerebral vessels is acquired from the petrous portion of the Internal Carotid Artery (ICA) to the level of the knee of the corpus callosum. Based on the images, the Circle of Willis (CoW) is classified as complete when both components are present: Bilateral anterior cerebral arteries (A1 segments), anterior communicating artery (A-com), bilateral posterior cerebral arteries (P1 segments), and bilateral posterior communicating arteries (P-com); or incomplete in the absence of any of these components.
The Cervical Vessels Magnetic Resonance Angiography (MRA) is acquired from the aortic arch to the base of the skull, with intravenous contrast injection in a 15-20 milliliter bolus. The degree of carotid stenosis is calculated from the images using the North American Symptomatic Carotid Endarterectomy Trial (NASCET) classification system.
For the statistical analysis of partial results, the scientific software (Statistical Package for the Social Sciences (SPSS) for Windows, version 20.0 ® ) was used, adopting a statistical significance level of p < 0.05. The Shapiro-Wilk test was employed to assess the distribution of variables. Descriptive analysis of quantitative variables was conducted by presenting measures of central tendency (mean or median) and dispersion (standard deviation or interquartile range). For variables with a normal distribution, mean and standard deviation were obtained, and for those with a non-normal distribution, median and interquartile range values were calculated. The agreement between intraoperative angiofluoresceinography and doppler within 24 hours was determined using Cohen's Kappa test.
Before CEA, all patients must be using acetylsalicylic acid at a dose of 100 milligrams per day for at least 5 days. Initially, monitoring is carried out for intra-arterial pressure (radial artery), electrocardiogram, pulse oximetry, and coagulation time. All procedures are performed with the patient under local anesthesia.
After positioning in the dorsal decubitus with the head slightly extended and turned to the contralateral side, an incision is made at the medial edge of the sternocleidomastoid muscle, extending from the lower neck to the tip of the mastoid process. Once the common carotid sheath is identified and before starting the dissection, the patient is heparinized with 1 milligram of heparin per kilogram of body weight. Heparinization is monitored by the coagulation time, which ideally should be over 200 seconds. Arterial dissection begins at the common carotid artery toward the bifurcation. After exposing the common, internal, and external carotid arteries and the superior thyroid artery (Figure 1), the internal carotid artery (ICA) occlusion tolerance test is performed, where the ICA is clamped for 2 minutes. During this time, the anesthesiologist tests the muscle strength of the contralateral upper and lower limbs, speech, vision, etc. If the patient tolerates ICA occlusion for 2 minutes, the operation proceeds normally. Patients who exhibit intolerance to ICA occlusion for less than 30 seconds are considered to have intolerance to the occlusion test, and the surgery is interrupted.
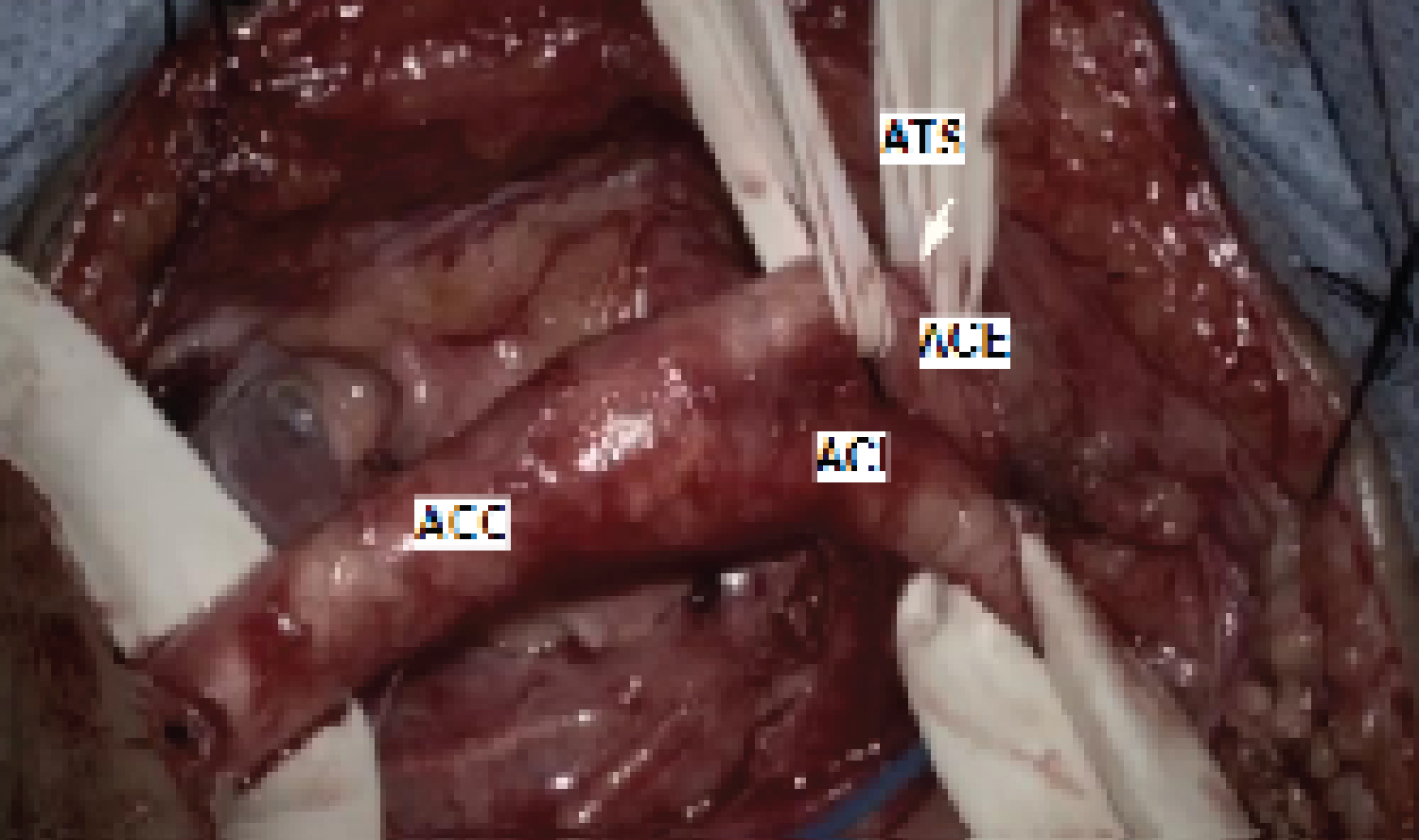 Figure 1: Exposure of the surgical field.
Figure 1: Exposure of the surgical field.
ACC: Common Carotid Artery; ACI: Internal Carotid Artery; ACE: External Carotid Artery; ATS: Superior Thyroid Artery.
View Figure 1
The arteriotomy begins at the common carotid artery and extends towards the internal carotid artery to the distal portion of the atheromatous plaque. The plaque is then progressively excised. Arteriorrhaphy is performed with continuous suturing using polypropylene suture (Prolene 6.0 ® ). Before closing the final stitches, the clamps are progressively removed and repositioned to allow blood flow to eliminate small fragments, clots, or air bubbles. Then, the process of definitive clamp removal begins: The first clamp removed is from the external carotid artery, followed by the common carotid artery clamp, allowing blood to flow towards the external carotid artery for about 2 to 3 minutes. Finally, the clamp on the internal carotid artery is removed. After completing the suturing, fluorescein is injected as a bolus of 5 milligrams per kilogram of body weight without dilution through venous access by the anesthetist, with the aim of documenting distal flow in the internal and external carotid arteries (Figure 2).
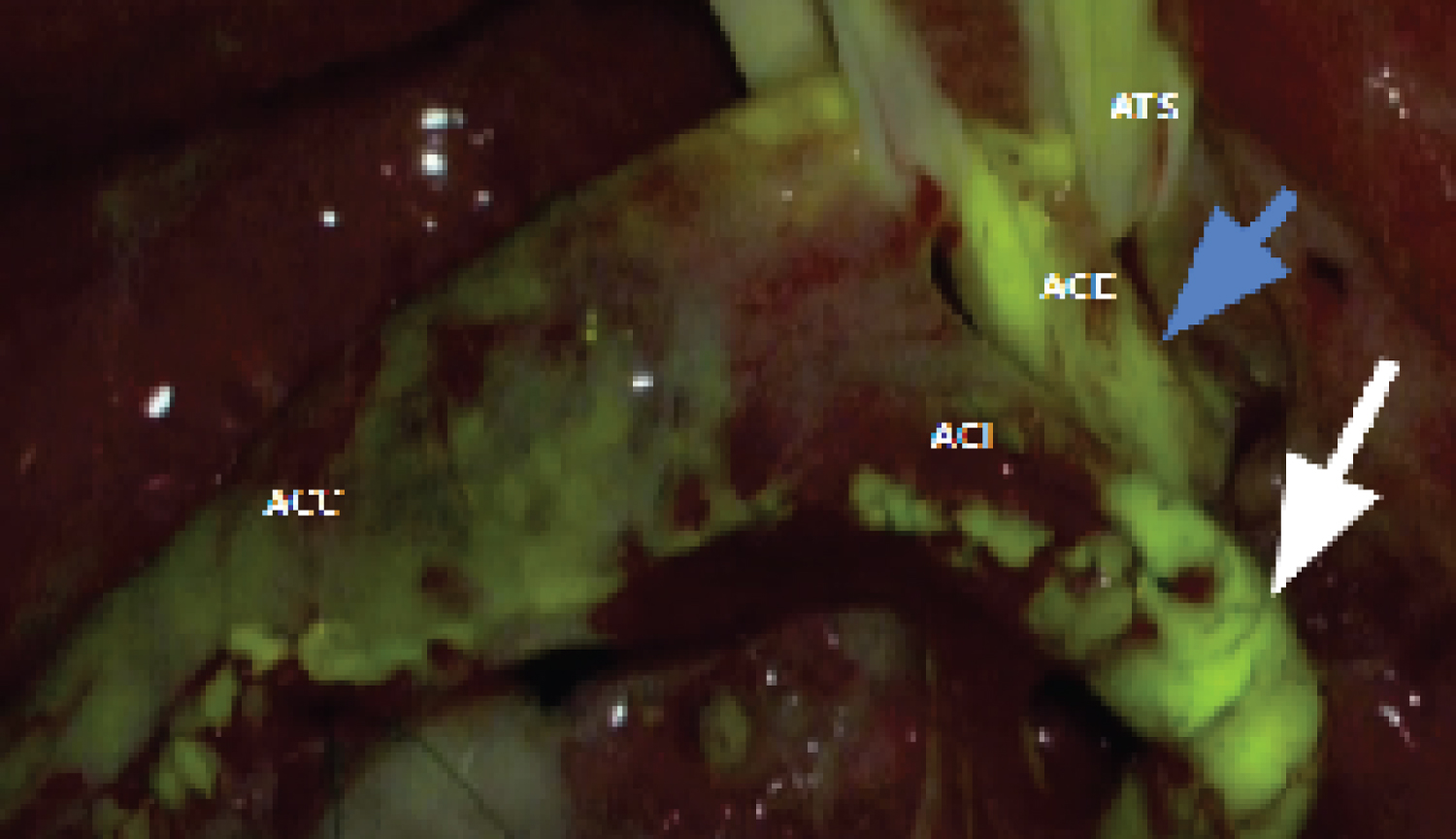 Figure 2: Fluorescence under the microscope light after fluorescein. Caption: ACC - common carotid artery; ACI - internal carotid artery; ACE - external carotid artery; ATS - superior thyroid artery. The white and blue arrows demonstrate the presence of distal flow in the internal and external carotid arteries, respectively.
View Figure 2
Figure 2: Fluorescence under the microscope light after fluorescein. Caption: ACC - common carotid artery; ACI - internal carotid artery; ACE - external carotid artery; ATS - superior thyroid artery. The white and blue arrows demonstrate the presence of distal flow in the internal and external carotid arteries, respectively.
View Figure 2
The coagulation time is then reassessed to evaluate the need for reverse heparinization. When the coagulation time exceeds 200 seconds, half of the heparin dose is reversed. If the coagulation time is less than 200 seconds, reversal is not performed. A drain with gentle suction is left in the subcutaneous tissue, and the wound is sutured layer by layer. At the end of the procedure, all patients are transferred to the intensive care unit for 24 hours with troponin curve monitoring every 6 hours, two collections, and then the drain is removed 12 hours after the procedure.
A bolus injection of fluorescein is administered at a dose of 5 milligrams per kilogram of body mass, undiluted, intravenously after arteriorrhaphy to assess distal flow in the ICA and ECA. The fluorescence module of the surgical microscope (Zeiss Pentero 800S ® ) is utilized, enabling real-time intraoperative videoangiography with three-dimensional reconstruction of the vessel.
The present study is ongoing; therefore, the results are based on a sample of 14 patients from March 2021 to July 2023, who underwent a total of 15 CEAs. The average age was 67.93 (± 7.75) years, with 71.4% (n = 10) being male. The most prevalent comorbidity among these patients was arterial hypertension, observed in 85.7% (n = 12), followed by dyslipidemia in 57.1% (n = 8), and diabetes in 50% (n = 50). One patient (7.1%) had other comorbidities, and 42.8% (n = 6) were smokers (Table 1).
Table 1: Characteristics of the studied population. View Table 1
Regarding symptoms, 71.4% (n = 10) of the patients were symptomatic, among whom 60% (n = 6) had ischemic strokes (AVCi), and 40% (n = 4) had transient ischemic attacks (AIT). Concerning the assessment of physical status according to the American Society of Anesthesiologists (ASA) classification, 57.1% (n = 8) were classified as ASA grade II, 21.4% (n = 3) as ASA grade III, and 21.4% (n = 3) as ASA grade IV. The PW was classified as complete in all 14 patients (Table 1).
All patients undergoing CEA were monitored in the intensive care unit for 24 hours, and the troponin curve yielded negative results (two collections with a 6-hour interval) following the study design.
Out of the 15 procedures, in 14 cases, videoangiography with fluorescein demonstrated distal flow in the carotid bifurcation. In one case, distal flow in the internal carotid artery was not documented. Choosing not to reopen the vessel, as the patient did not develop neurological deficits. The absence of flow in the internal carotid artery in this patient was confirmed with the control Doppler performed within the first 24 hours after CEA. The Kappa test indicated perfect agreement between the fluorescein test and the Doppler performed within 24 hours (k = 1.000; p < 0.001; agreement: 100%) (Table 2).
Table 2: Concordância entre Videoangiografia com fluoresceína intraoperatória e Doppler de até 24 horas. View Table 2
The Doppler within 24 hours, using the cutoff point from the consensus of radiologists and ultrasonographers, 6 revealed that 14 (93.3%) out of the 15 procedures performed were reported as the absence of plaque, and 1 (6.7%) was reported as 100% occlusion (Table 3). For this patient with 100% occlusion, the intraoperative videoangiography with fluorescein was negative.
Table 3: Doppler within 24 Hours. View Table 3
During the period from March 2021 to July 2023, there was no loss of follow-up or participant withdrawal. There were no deaths, ischemic strokes, or acute myocardial infarctions, even in the patient who had thrombosis of the internal carotid artery (ACI) at the end of the procedure, documented by the absence of flow in videoangiography with fluorescein.
The Doppler results at 3 months for the 15 procedures remained similar to the Doppler within 24 hours.
Carotid stenosis is one of the main causes described for ischemic stroke (IS), pathologically characterized by atherosclerosis [1]. Carotid endarterectomy (CEA) is considered the treatment of choice in terms of efficacy and safety, barring contraindications. The majority of post-procedural thromboembolic events can be avoided if detected and treated early [7-9]. However, it has been demonstrated that up to 7% of CEA procedures require immediate surgical correction due to technical issues [10,11].
Thus, intraoperative tests to detect vessel patency and intra-arterial thrombus after CEA are crucial to avoid complications. Despite arteriography being the gold standard for vascular examinations, it has some disadvantages, such as being an invasive procedure and requiring the use of radiation-loaded fluoroscopy for the patient [12]. Doppler was introduced as a non-invasive method to assist in the intraoperative assessment of arterial flow; however, the exam is operator-dependent [9], and it presents technical limitations that may hinder the evaluation of distal flow in the cervical internal carotid artery (ICA).
In light of the above, diagnostic tools to identify the absence of distal flow and the presence of thrombus after arteriography must be accurate and prompt. Based on recent publications, the use of fluorescent markers in cerebral microvascular surgery has been gaining prominence for this modality. According to Bozkurt, et al., [13], indocyanine green in bolus at the end of the procedure correctly assessed flow in 38 (86.4%) out of 44 procedures; however, the high cost of indocyanine green may be a barrier to its use in public health.
Fluorescein, another tracer gaining prominence, was studied by Świątnicki, et al. , [14]. Their cohort study showed a significant difference (p < 0.005) in the absence of residual neck in cerebral aneurysms where clipping was aided by intraoperative videoangiography with fluorescein compared to the control where it was not used. Thus, videoangiography with fluorescein in vascular microsurgeries may become a tool capable of detecting thrombosis and stenosis of the common carotid artery and its terminal branches after arteriorrhaphy. Partial results from the ongoing study confirm this hypothesis, with 14 (93.3%) out of 15 procedures showing flow in both ICA and ECA, results consistent with Doppler within 24 hours.
Chaturvedi, et al., [15] state that the safety of carotid endarterectomy arises from the reduction of complications risk, mainly from minimizing the risk of perioperative stroke. For the surgery to be effective, the rate of IS, myocardial infarction (MI), and death within 30 days should be < 3% for asymptomatic patients and < 6% for symptomatic patients. From the analysis of partial results from the ongoing study, no complications occurred, even in the patient with carotid occlusion, where the decision was made not to reopen the vessel as no neurological deficit developed. Collateral circulation in this patient was previously studied by 3D TOF MRA and was found to be complete, corroborating with Paraskevas, et al., [16] statement that the safety of CEA also depends on the identification of preoperative risks, such as incomplete anatomy of the Circle of Willis. Regarding the presented results, it's important to consider that the study is ongoing.
Based on the partial results presented, we conclude that intraoperative videoangiography with fluorescein during carotid endarterectomy is an innovative and as yet undescribed technique in the literature. There is an expectation that upon study completion, it will emerge as a safe and cost-effective technique, potentially having a significant impact on both public and private healthcare services.
Own financing.
Dantas Mageste Ferreira provided guidance at all stages of the research. Jéssica Aguilar da Silva, Vitor de Deus da Rocha Ribeiro Gonçalves and Lano de Sousa Moreira wrote the main manuscript text. Taianne Fiore Schumann made tables and prepared figures. All authors reviewed the manuscript.