Meningioma presenting as ischemic stroke is very rare. We report a case of left medial sphenoid wing meningioma presenting with cerebral infarction due to occlusion of left middle cerebral artery (MCA) in a 36-year-old female. We discuss the incidence, pathophysiology, management and review of literature of intracranial meningioma presenting as ischemic stroke.
Ischemic stroke, M1 segment, MCA, Meningioma, Surgery, Sphenoid wing
A 36-year-old right-handed female without significant medical comorbidity was brought to Emergency and Accident (A&E) Department with the history of sudden onset of severe headache followed by vomiting and unconsciousness for 24 hours. She was referred to our center after initial resuscitation at another hospital. On arrival, her GCS was 11 with eye opening three, motor score 5 and verbal score three. He was hemiplegic on right side. There was anisocoria with left pupil dilated, fixed and not reacting to the light; her right pupil was normal and reacting to the light. Contrast MRI of the Brain showed left sided homogenous enhancing extra axial mass attached to medial sphenoid wing and planum sphenoidal area encasing the left internal carotid artery (ICA) and M1 segment of left MCA (Figure 1A, Figure 1B, Figure 1D, Figure 1E and Figure 1F). MRA showed complete occlusion of left MCA at M1 segment (Figure 1G). T2/FLAIR image revealed hyperintense area in left MCA territory suggestive of infarct (Figure 1C).
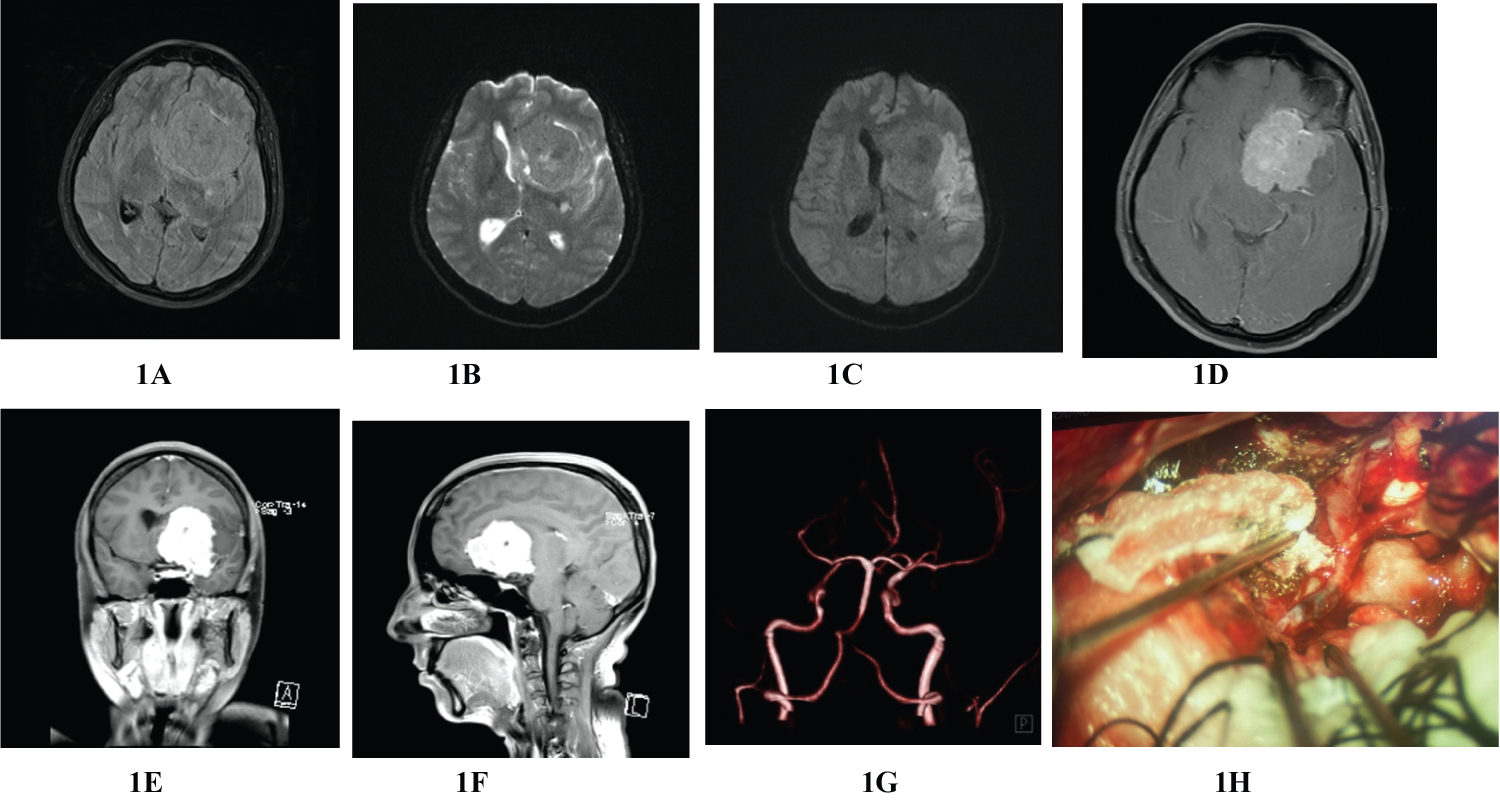 Figure 1: Preoperative MRI of brain; T1W & T2W images showing large solid mass at left frontotemporal region with midline shift (1A and 1B), T1/FLAIR image depicting left frontotemporal mass with MCA territorial infarct (1C), contrast MRI of brain with axial, coronal and sagittal sections showing large extraaxial homogenously enhancing left frontotemporal mass attached to medial sphenoid, clinoid and planum sphenoidal areas (G-F), MRA revealing total occlusion of left MCA at A1 segment (G), intraoperative picture showing intraluminal thrombus (black segment) at left M1 segment causing total occlusion of artery (H).
View Figure 1
Figure 1: Preoperative MRI of brain; T1W & T2W images showing large solid mass at left frontotemporal region with midline shift (1A and 1B), T1/FLAIR image depicting left frontotemporal mass with MCA territorial infarct (1C), contrast MRI of brain with axial, coronal and sagittal sections showing large extraaxial homogenously enhancing left frontotemporal mass attached to medial sphenoid, clinoid and planum sphenoidal areas (G-F), MRA revealing total occlusion of left MCA at A1 segment (G), intraoperative picture showing intraluminal thrombus (black segment) at left M1 segment causing total occlusion of artery (H).
View Figure 1
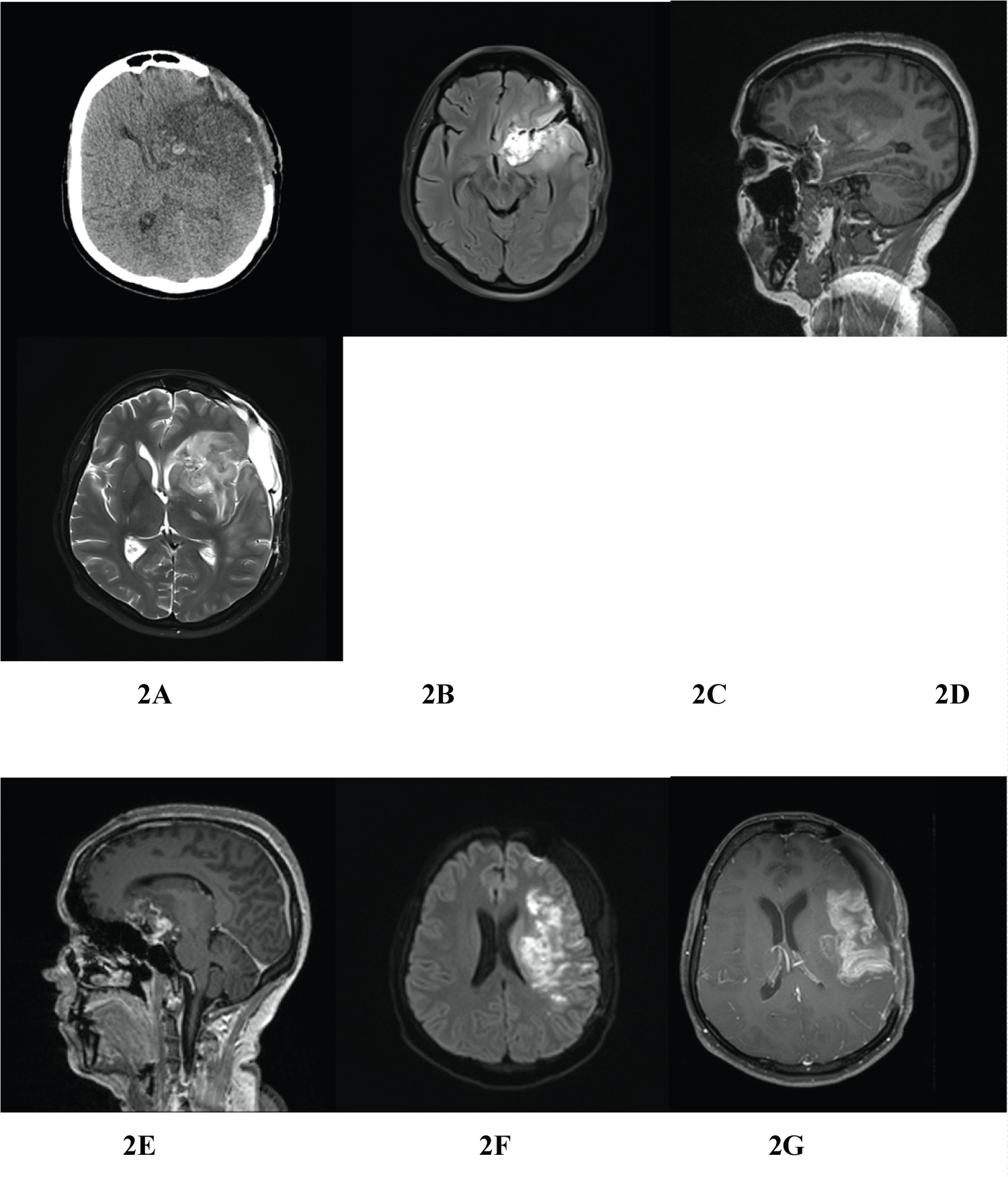 Figure 2: Postoperative images; plain CT scan of brain shows left MCA infarct and no evidence of residual tumor (A), contrast MRI of brain showing edema in and around the operated area with left MCA infarct and no evidence of residual tumor (B-G).
View Figure 2
Figure 2: Postoperative images; plain CT scan of brain shows left MCA infarct and no evidence of residual tumor (A), contrast MRI of brain showing edema in and around the operated area with left MCA infarct and no evidence of residual tumor (B-G).
View Figure 2
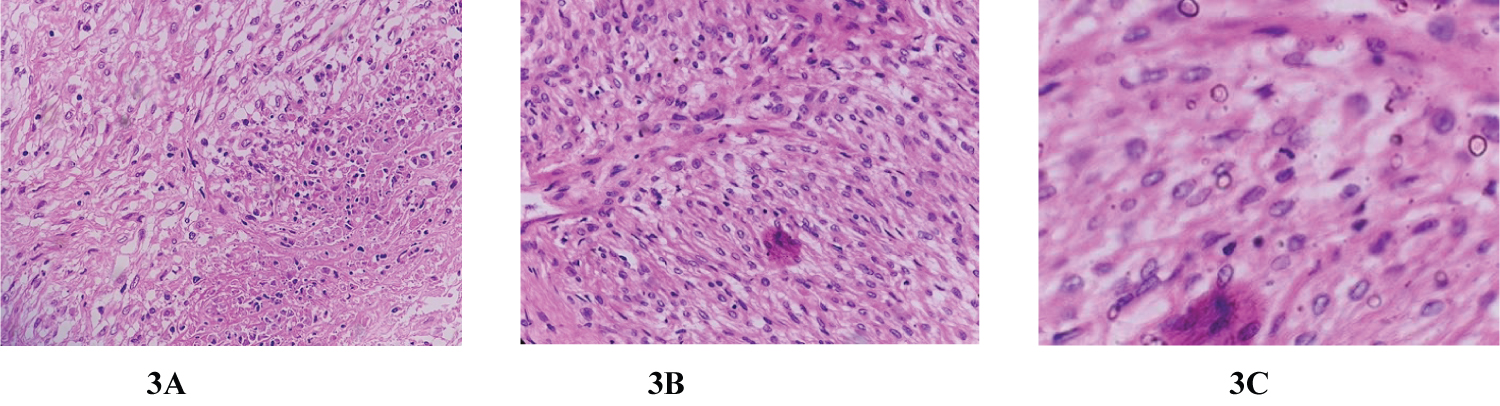 Figure 3: Histopathological features. (H&E ×200 (A), H&E ×400 (B) and H&E ×800(C)); there are increased areas of cellularity and foci of necrosis; tumor tissues are composed of meningoepithelial cells arranged in whorls, lobules and diffuse patternless sheets of round to oval to plump spindle cells; rare mitotic figures and psammomatous calcification are identified. All these microscopic features are suggestive of atypical meningioma (WHO grade II).
View Figure 3
Figure 3: Histopathological features. (H&E ×200 (A), H&E ×400 (B) and H&E ×800(C)); there are increased areas of cellularity and foci of necrosis; tumor tissues are composed of meningoepithelial cells arranged in whorls, lobules and diffuse patternless sheets of round to oval to plump spindle cells; rare mitotic figures and psammomatous calcification are identified. All these microscopic features are suggestive of atypical meningioma (WHO grade II).
View Figure 3
She underwent emergency left pterional craniotomy, large bone flap was raised, and lateral sphenoid ridge was shaved for better visualization of operating area. Gross total excision (Simpson grade III) of meningioma was achieved. Intraoperative findings were solid, hard and vascular extra axial mass attached to left clinoidal and planum sphenoidal areas and had encased ICA and M1 segment; there was complete occlusion at left M1 segment by thrombus and was confirmed by TCD (transcranial doppler) and ICG (indocyanine green) angiography. There was tremendous amount of brain edema during procedure so bone flap was removed and laxed duroplasty was done. Post-operatively patient was kept on ventilator for 72 hours and then extubated. The size of the left pupil regressed just after surgery. On 3 rd post op day, patient was fully conscious but she remained right sided hemiplegic and aphasic. Postoperative images (CT/MRI) showed no residual tumor except areas of brain edema and infract (Figure 2A, Figure 2B, Figure 2C, Figure 2D, Figure 2E, Figure 2F and Figure 2G). After three weeks of vigorous physio and speech therapy she was able to walk with support and spoken few words. Histopathology report was described as increased areas of cellularity, foci of necrosis, composed of meningoepithelial cells arranged in whorls pattern with occasional mitotic figures which are suggestive atypical meningioma (WHO Grade II) (Figure 3A, Figure 3B and Figure 3C). Since patients had Simpson grade III excision (gross total excision) we decided to have close observation with regular follow up and did not recommend for either radio or chemotherapy.
Meningiomas are 2 nd most common primary CNS tumor of brain after glioma, accounting about 13-26% of all primary brain tumor [1]. They are more common among females and may be linked with female hormones (estrogen and progesterone) [2,3]. Twenty five percent of intracranial meningiomas are located at skull base and reported ratio between calvarial and skull base meningioma is 2.3:1 [1]. Skull base meningiomas are usually originated at sellar, parasellar, cavernous, frontobasal, temporobasal, sphenoid wing or petroclivus regions. Meningiomas originated in these locations often involve ICA and its branches and but rarely compromise cerebral blood flow [4].
Common presentations of intracranial meningiomas are features of raised intracranial pressure (ICP), seizure, focal neurological deficit and cranial nerve palsies and they are usually insidious onset. Some meningiomas are asymptomatic and are only detected when patients are investigated for other medical problem. Occasionally patients with intracranial meningioma may present acutely with intratumoral bleed and only 2% of meningiomas are associated with spontaneous intracranial hematoma [5,6]. There are many anecdotal case reports of intracranial meningiomas presenting as TIA (transient ischemic attack) [7-11]. However, Intracranial meningiomas presenting as acute Ischemic stroke or infarction is very rare and only 7 cases have been reported in literature to this date [4,12-16]. From 2007 to 2022 we operated on 266 patients with intracranial meningiomas at our institute and we found only one patient (present case report) presenting as acute stroke making a incidence of 0.38% (1/266) in our series. In 2003, Komotar, et al. published two cases of meningiomas presenting as acute stroke and in his series of 1617 meningiomas he found 3 cases of meningiomas with acute stroke due to ICA compression, accounting 0.19% incidence (3/1617) [4].
Meningioma is usually slow growing, non-invasive tumor, and arteries with high flow like ICA, MCA and ACA may be less likely to compromise than cortical veins and dural sinuses. However, occurrence of stroke in patients with skull base meningiomas have been described in literature and believed to be due to either direct vascular compression or vascular infiltration by tumor or indirectly by coagulation disorder [4,12-17]. It has been found that progesterone receptors are expressed in 50 to 80% of human meningioma and size of the meningioma may be accelerated during second half of pregnancy but whether it has any association to meningioma with acute brain infarct is not documented [1,2].
In all these seven reported cases there were 5 male and 2 female and age ranged from 31 to 58 years; 5 meningiomas were located at sphenoid ridge, 1 cavernous sinus and 1 olfactory groove. Occluded arteries were ICA (3), M1 segments (3) and A2 segment (1) and most common infarcted areas were MCA (6) and ACA (1) territories [4,12-16] (Table 1).
There is no guideline regarding the management of intracranial meningiomas with acute cerebral infarct. Treatments documented in these seven reported cases were individualized based on time of arrival, GCS at presentation, location of meningioma and availability of expertise and technology. If patient comes late with compromised GCS and features of coning like in our case, there is no other choice except decompressive craniectomy and excision of tumor as a lifesaving procedure [13,16]. But if patient arrives within time frame, if GCS is normal and there are no signs of coning emergency endovascular stenting or mechanical thrombectomy would be recommended followed by excision of tumor at next sitting [12] or EC-IC Bypass followed by excision of tumor in a single sitting is another treatment option [14].
Radiosurgery has been used in inoperable cavernous sinus meningiomas with ICA occlusion due to tumor infiltration [4,17].
Regarding outcome of these seven reported cases there was no procedure related mortality, however all these patients had some residual neurological deficit postoperatively no matter what treatment they were opted. In our case, patient survived after surgical intervention but remained severely disabled.
Intracranial meningioma with acute cerebral infarct is very rare and usually happens due to occlusion of ICA and its distal branches by skull base meningiomas mostly at sphenoid ridge. Most commonly involved arteries were M1 segment of MCA and ICA producing MCA territorial infarct. Here we report a case of medial sphenoidal wing meningioma presenting as stoke secondary to complete occlusion of left M1 segment of ICA.