We report a case of rapidly expanding de novo cavernoma in the sensory cortex causing/producing/evoking cervicobrachialgia.
A 32-year-old female presented with neck pain radiating to the ulnar forearm along with occasional tingling sensations. Three years earlier, she had suffered from left-sided cerebellar infarction caused by dissection of the left vertebral artery. The dissection was provoked by manual therapy administered by a certified physio-therapist. Antiplatelet treatment with Aspirin 100 mg/day was established. One year later, she experienced tingling in the left side of her face. At the time, intracerebral micro-hemorrhage in the right sensory cortex was seen on cranial computed tomography (CCT) and Aspirin was discontinued. A routine follow-up magnetic resonance imaging (MRI) two years later showed an increase of the hemorrhagic lesion, suggestive of a de novo cavernous hemangioma. In a follow-up MRI three months later the lesion had almost tripled in size and the patient was advised to consult a neurosurgeon. Days before the neurosurgical consultation, previously mild pain that irradiated from the right side of her neck to the ulnar side of the right arm increased sharply. Following microsurgical excision of the cavernoma, the symptoms decreased gradually. An MRI of the cervi-cal spine was normal with no signs of disc herniation or nerve compression.
On rare occasions, lesions in the respective area of the somatosensory cortex may cause cervicobrachialgia.
Cavernous angioma, Cervicobrachialgia, Sensory cortex
Cerebral cavernous angiomas (synonym: cerebral cavernous malformations; cavernomas) are the third most common cerebral vascular malformation after developmental venous anomaly and capillary telangiectasia [1]. Cavernomas are comprised of clusters of tightly packed, abnormally thin-walled blood vessels that displace normal neurological tissue in the brain or spinal cord [2]. Three gene mutations (CCM1, CCM2, and CCM3), which can either be inherited or occur sporadically, have been associated with the formation of cavernomas [3].
Intracerebral hemorrhage is the initial complication in 30% of cases [4,5]. Superficial cavernomas are associ-ated with a lower risk of hemorrhage than those located in the depth. Patients with asymptomatic cavernomas with typical imaging findings are usually managed conservatively with serial imaging studies. Symptomatic patients may present with acute headache, epilepsy and acute or subacute neurological deficits owing to overt intra or extralesional hemorrhage [6].
We report the case of a 32-year-old female who suffered from symptoms classified as cervicobrachialgia that were caused by a rapidly growing de novo cavernoma located in the post central gyrus.
A 32-year-old left-handed female presented with neck pain in the area of the lateral triangle on the right, radi-ating to the ulnar forearm. The pain went along with occasional tingling sensations and was increased by rais-ing the right arm. The symptoms were independent of head movements. There were no complaints of head-ache, nausea or vomiting.
In September 2017, the patient had suffered from left-sided cerebellar infarction caused by dissection of the left vertebral artery following manual therapy administered by a certified physiotherapist. Thereafter, an-tiplatelet treatment with 100 mg/day of Aspirin was started. The patient was oligosymptomatic until November 2018, when she reported an isolated tingling sensation on the left side of her face. At that time, intracerebral micro-hemorrhage was seen on the CCT and Aspirin was discontinued.
In January 2020, the patient reported minor holocranial headache (VAS 3/10) for the first time. An MRI showed a hyperdense lesion in the left postcentral gyrus which was classified as a small cavernous angioma by a radiologist. In March, the patient reported painful dysesthesia in the right lateral cervical triangle, radiat-ing over the lateral upper arm to the ulnar forearm. The tendon of the trapezius muscle was palpably and even visibly tense on the right side. Another follow-up MRI was performed on April 20, which showed an increase in size of the lesion to about three times the previous size.
On examination, the left-handed patient showed a prominent and tense superior tendon of the trapezius mus-cle on the right side. Algophobic weakness of the triceps muscle on the right side. During examination the pain was worsened by both actively and passively raising the right arm. All symptoms were independent of head movements. No other motor or sensory deficit was identified.
In December 2017, apart from showing the circumscript postischemic lesion in the left cerebral hemisphere, an MRI of the head did not reveal any significant abnormalities (Figure 1A). On January 22, 2020, a small, contrast-enhancing hemorrhagic lesion could be seen in the left postcentral gyrus, more precisely in the soma-tosensoric area representing the right arm (Figure 1B and Figure 2). In April 2020, the lesion had tripled in volume (Figure 1C).
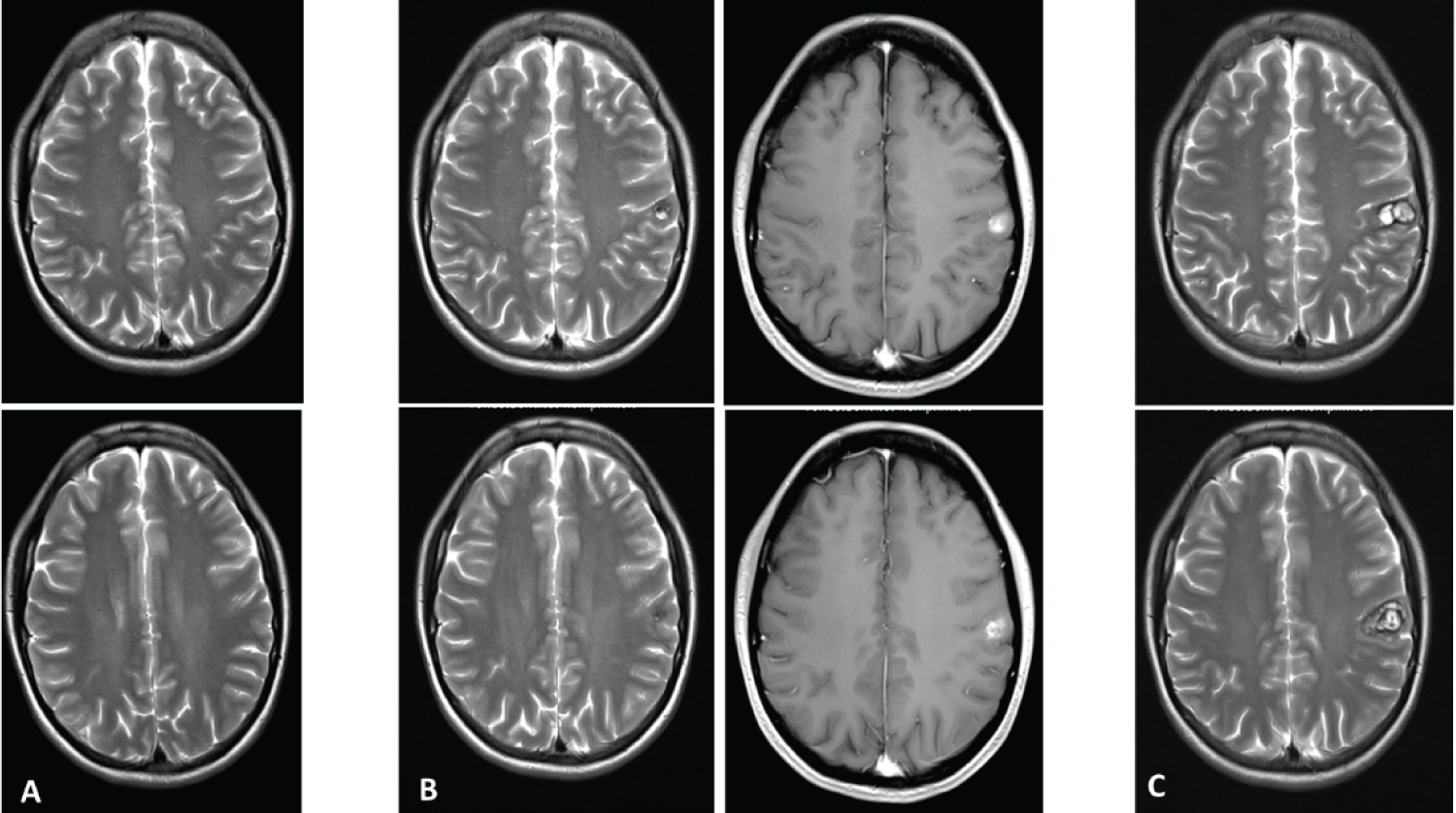 Figure 1: (A) T2-weighted MRI taken in Dec 2017, showing no lesion in the somatosensory cortex; (B) T2- and post-contrast T1-weighted images taken in Jan 2017. De novo cavernoma in the left somatosensory cor-tex; (C) Follow-up T2-weighted MRI three months later, the lesion had tripled in volume and the patient suf-fered from left-sided cervicobrachialgia and dysesthesia. View Figure 1
Figure 1: (A) T2-weighted MRI taken in Dec 2017, showing no lesion in the somatosensory cortex; (B) T2- and post-contrast T1-weighted images taken in Jan 2017. De novo cavernoma in the left somatosensory cor-tex; (C) Follow-up T2-weighted MRI three months later, the lesion had tripled in volume and the patient suf-fered from left-sided cervicobrachialgia and dysesthesia. View Figure 1
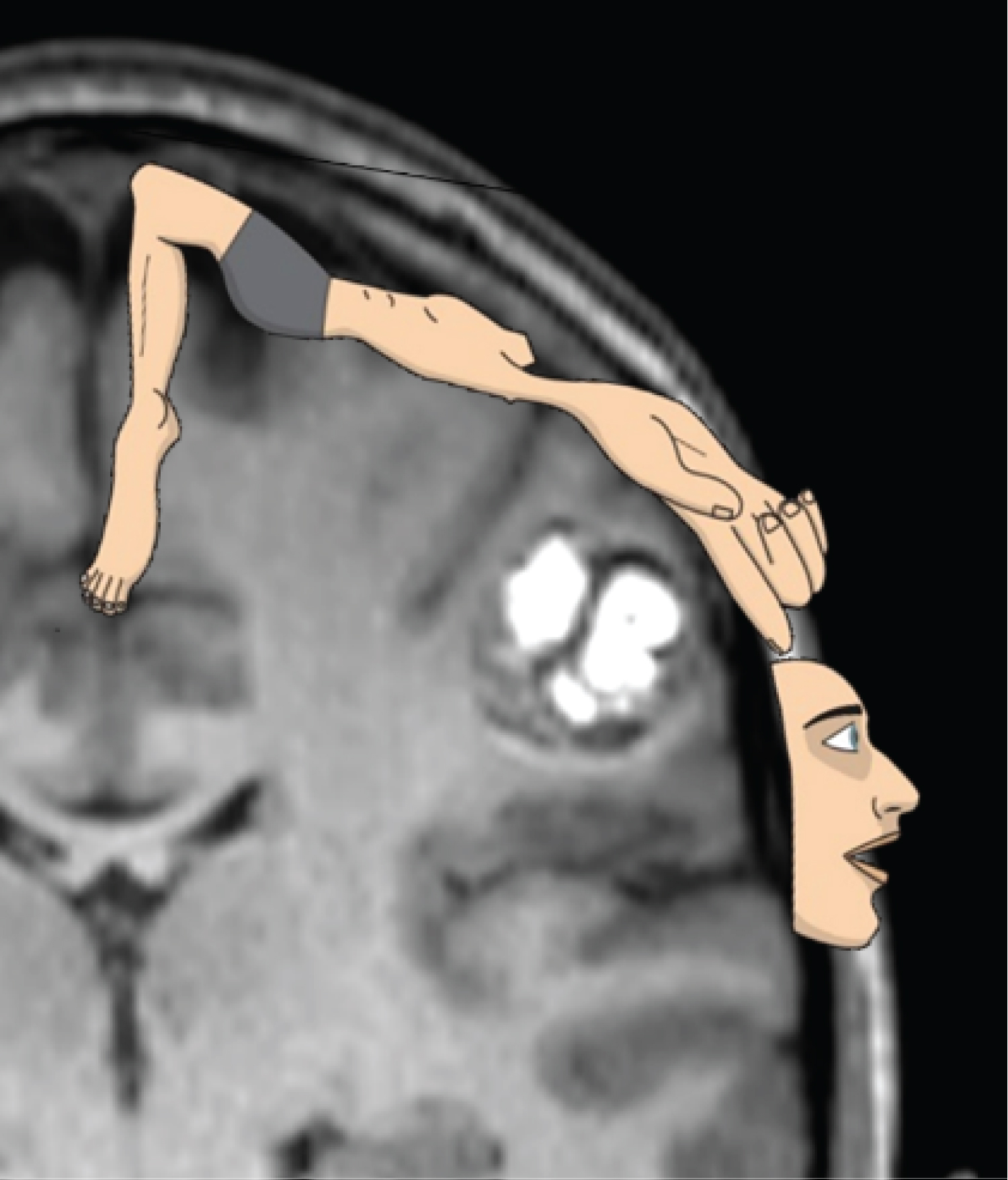 Figure 2: Coronal section of the T1-weighted, contrast-enhanced MRI showing the cavernoma before surgery. On overlay of the schematic homunculus on the somatosensory cortex shows the lesion to be in the area cor-responding to the sensory representation of the arm and the hand.
View Figure 2
Figure 2: Coronal section of the T1-weighted, contrast-enhanced MRI showing the cavernoma before surgery. On overlay of the schematic homunculus on the somatosensory cortex shows the lesion to be in the area cor-responding to the sensory representation of the arm and the hand.
View Figure 2
The MRI of the cervical spine did not show any signs of compression of neural structures (Figure 3).
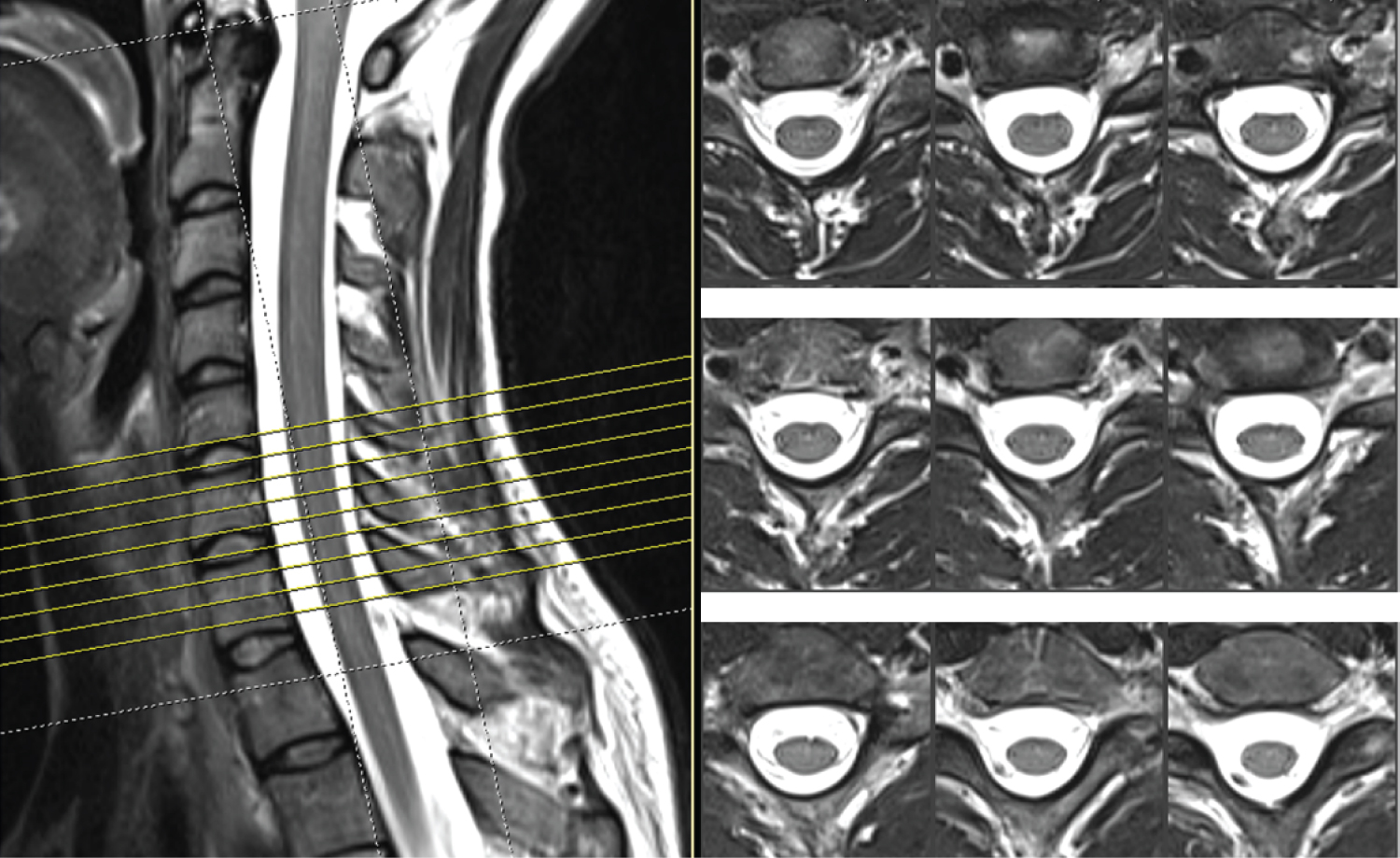 Figure 3: Cervical spine MRI of the patient showing normal radiological findings.
View Figure 3
Figure 3: Cervical spine MRI of the patient showing normal radiological findings.
View Figure 3
With the patient in supine position, a left parietal craniotomy was performed under image-guidance. A micro-surgical approach with a corticotomy at the site where the lesion was closest to the surface was chosen, and intraoperative ultrasound was employed as a second guidance system. For additional safety, reversal soma-tosensensory potentials were measured before the corticotomy was carried out. The cavernoma was anatomi-cally dissected from the surrounding somatosensory cortex and removed entirely without complications.
Histological sections of the cavernous hemangioma showed numerous back-to-back, partly capillary, partly cavernous, dilated blood vessels with abundant surrounding hemosiderophage accumulations and moderate lymphohistiocytic inflammation caused by relapsed hemorrhages and surrounding reactive piloid astrogliosis. No atypia was seen in endothelial cells or the pericytes (Figure 4).
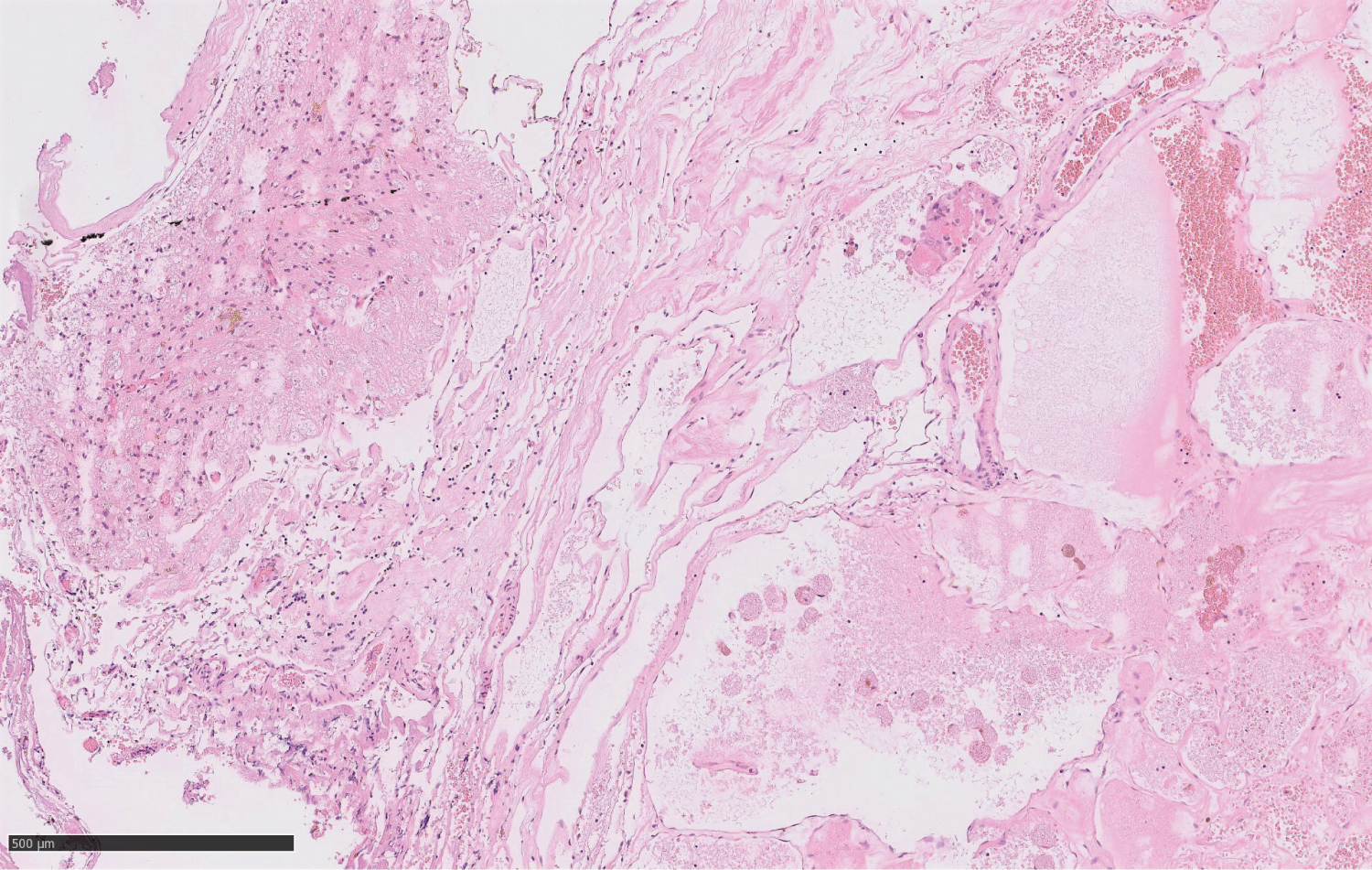 Figure 4: Hematoxylin-Eosin stained histological section through the surgical specimen of the lesion showing the typical features of a cavernoma with multiple, partly capillary, partly cavernous dilated blood vessels with abundant surrounding hemosiderophage accumulations and moderate lymphohistiocytic inflammation (WHO grade I).
View Figure 4
Figure 4: Hematoxylin-Eosin stained histological section through the surgical specimen of the lesion showing the typical features of a cavernoma with multiple, partly capillary, partly cavernous dilated blood vessels with abundant surrounding hemosiderophage accumulations and moderate lymphohistiocytic inflammation (WHO grade I).
View Figure 4
Postoperatively, the cervicobrachialgia resolved gradually and seized completely one month later. An MRI at three months post-surgery showed hemosiderin residuals in the area of previous surgery. Contrast-enhanced scans did not reveal any remnant of the cavernoma.
Cavernomas are more common type of intracerebral vascular lesions than is generally suspected. They are comprised of clusters of tightly packed, abnormally thin-walled, small blood vessels that displace normal neu-rological tissue in the brain or spinal cord [1,7]. In recent literature, the prevalence of cavernomas has been reported to be between 0.1% and 0.8% [3,8].
In most cases, cavernomas occur sporadically with isolated lesions. The familial form is characterised by mul-tiple lesions with an autosomal dominant mode of inheritance. Cavernomas have also been long known to cal-cify and to develop 'de novo' from microscopic arteriovenous lesions [9].
Histologically, the defective blood vessels in cavernomas lack pericytes and tight and adherent junctions re-sulting in a single layer lining consisting of endothelial cells. This leads to the impairment of the blood-brain barrier [10].
In the brain and spinal cord, cavernomas can be fragile and lead to bleeding, causing hemorrhagic stroke, sei-zures, and neurological deficits. They can range in size from a few fractions of an inch to several inches in diameter, depending on the number of blood vessels involved. Potential risk factors for hemorrhage include sex (females > males), age, lesion location, size, trauma, perfusion, and prior hemorrhage [11,12].
Common clinical manifestations are intralesional or extralesional intracerebral hemorrhage with or without focal neurological deficits and seizures [8,13].
In the case presented here, clinical symptoms included radicular pain and dysesthesia in the upper limb, closely resembling cervicobrachialgia caused by degenerative lesions of the cervical spine. This was particu-larly suggestive because the patient had a history of neck pain that had been treated by manual therapy years before. Tragically, the physiotherapeutical intervention at that time led to dissection of the vertebral artery and consecutive embolic cerebellar infarction.
Cervicobrachialgia is a term that describes pain and stiffness of the cervical spine with symptoms in the shoulder girdle and upper extremity [14]. It can be associated with tingling, numbness or discomfort in the arm, upper back and upper chest with or without an associated headache.
Our patient presented with all these symptoms. If it was not for her medical history that included the cerebel-lar ischemia which had been monitored through a follow-up MRI, an intracerebral cause of her symptoms would probably not have been suspected primarily. Diagnostic examination would probably have stopped at cervical imaging. Physical therapy might have been initiated and - in light of the patient's vulnerability with respect to vertebral artery dissection may have led to yet another complication.
After having encountered such an unusual clinical constellation, our own awareness to the differential diag-noses of cervicobrachialgia has heightened. If the pain cannot be provoked by cervical movements, and if there is no clear radicular distribution of sensory or motor symptoms, an intracerebral lesion should be sus-pected. These lesions can be located in or around the somatosensory cortex [15] and may include various pa-thologies.
If a cavernoma is suspected, magnetic resonance imaging of the brain is the best radiological examination to detect these lesions. The cranial MRI of our patient showed that the 'de novo' cavernoma was located in the postcentral gyrus, in the somatosensory area representing the right arm and hand (Figure 2). While averaged data of how incoming sensory information from the human body is represented in the postcentral cerebral cortex have long been aggregated into a 'sensory homunculus' (Figure 2), individual sensory representation may vary. Since the tension of our patient's trapezius muscle was relieved after removal of the cavernoma, we suggest that the sensory area of the neck was also involved by the lesion.
On rare occasions, cervicobrachialgia can be caused by lesions in the postcentral cortex where sensory infor-mation from the contralateral upper limb and side of the neck is processed.
Even in a patient with a previously inconspicuous cerebral MRI, a 'de novo' cavernoma can rapidly increase in volume and lead to cervicobrachialgia, a symptom complex normally only found in cervical spine lesions. In patients presenting with cervicobrachialgia where no cervical abnormalities can be detected, cerebral cav-ernoma should be considered as a differential diagnosis and consequently excluded through a cerebral MRI.