Chordoid meningioma is an uncommon histopathological type of meningioma, frequently associated with Castleman's syndrome. Histologically, chordoid meningiomas are similar to chordomas. Because of their high proliferative index, they present aggressive biological behavior and high risk of postoperative recurrence. We report a case of choroid meningioma in adult patient without Castleman's syndrome manifestation. As its chordoid features is related with a rapid recurrence after incomplete removal, meticulous histopathological examination is crucial for the adequate postoperative treatment plan.
Chordoid meningioma, Mitotic index, Castleman's disease
Meningioma is the most common benign intracranial tumor, representing 13-36% of all primary central nervous system neoplasms. Among meningiomas 90% of tumors are benign [1,2]. Starting form 2007 all meningiomas with cortical invasion became considered as grade II [3]. This increased the rate of these meningiomas from 5% to 20-35% [3]. Among grade II group, besides atypical and clear cell meningiomas, the rare type of these tumors constitutes chordoid meningiomas (CM). CMs represent approximately less than 1% of intracranial meningiomas [4-6]. The term chordoid meningioma was initially presented by Kepes, et al. [7] who defined it as having a chordoma-like histologic appearance with a clustering of tumor cells. We report a 71-years-old patient with chordoid meningioma in parasagittal region infiltrating the superior sagittal sinus and motor cortex of the left hemisphere.
A 71-years-old non-smoking right-handed woman, presented with two months history of progressive left leg paresis. Cranial magnetic resonance imaging (MRI) revealed a 3 × 4 × 4 cm mass in the left occipital region with superior sagittal sinus infiltration (Figure 1A and Figure 1B).
Two weeks after the initial presentation, she underwent the left fronto-parietal craniotomy. Considering the superior sagittal sinus infiltration by the tumor, advanced age of the patient, a subtotal tumor resection was undertaken. Small tumor remnants within the sinus cavity were coagulated thoroughly. The infiltration of the arachnoid by the tumor increased the cerebral cortex injury. Left lower limb paresis progression was observed immediately after the procedure, and it subsequently diminished during the rehabilitation. Control magnetic resonance imaging carried out one month after surgery confirmed tumor remnants within the superior sagittal sinus cavity (Figure 1C and Figure 1D). The patient was consulted by oncologist and stereotactic radiotherapy (Cyberknife) was applied to irradiate the remnants of the tumor within the superior sagittal sinus. No tumor tissue progression was observed six months after surgery (Figure 1E and Figure 1F).
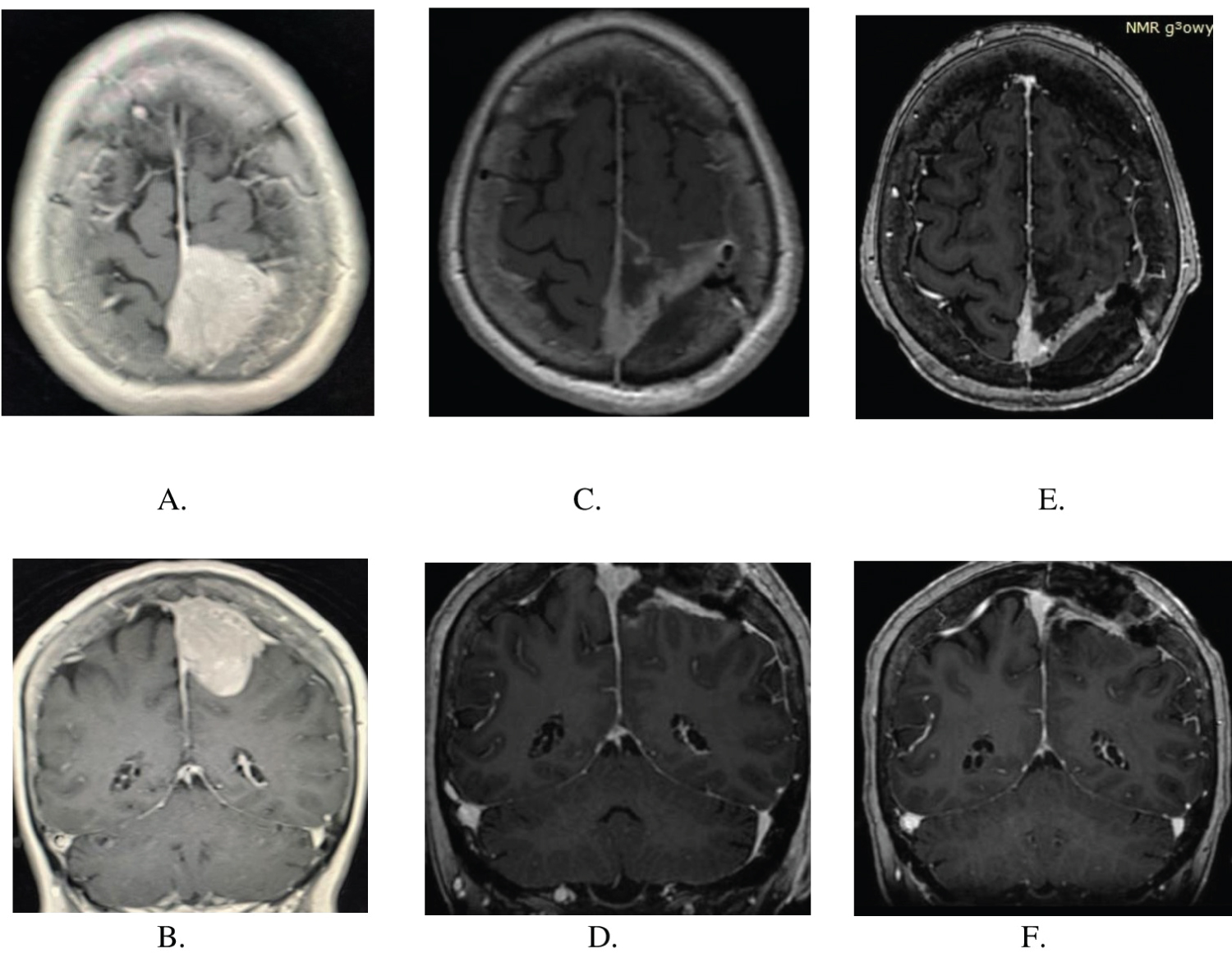 Figure 1: (A,B) MRI scans showing Choroid Meningioma in axial and sagittal plane before surgery; (C,D) MRI scans showing tumor remnants in the superior sagittal sinus one month after surgery, before Gamma Knife therapy; (E,F) MRI scans six months after surgery, recently after Gamma Knife therapy.
View Figure 1
Figure 1: (A,B) MRI scans showing Choroid Meningioma in axial and sagittal plane before surgery; (C,D) MRI scans showing tumor remnants in the superior sagittal sinus one month after surgery, before Gamma Knife therapy; (E,F) MRI scans six months after surgery, recently after Gamma Knife therapy.
View Figure 1
The samples tissue from neurosurgical procedure were fixed in 10% buffered formalin and paraffin embedded. The specimens were stained with hematoxylin-eosin and mucicarmine methods. Immunohistochemical studies were performed with antibodies to epithelial membrane antigen (EMA) (1:200, Leica clone GP1,4), cytokeratin, CK (1:50, DACO clone AE1/AE3), glial fibrillary acid protein, GFAP (1:300, Bio-Rad, clone 1B4), Ki-67 (1:75, Invitrogen, clone SP6). The sections were examined and photographed.
Neuropathological examination revealed that samples of the tumor were composed of cords of round, ovoid and epithelial cells, embedded in a prominent myxoid background (Figure 2A, Figure 2B and Figure 2D). Sometimes, mild nuclear atypia was focally present. The conventional meningothelial or transitional morphology was visible in small fields (Figure 2C). Immunohistochemical staining showed positive staining for epithelial membrane antigen (EMA) (Figure 2E). The Ki-67 labeling index was about 8% (Figure 2F). The tumor cells were negative for glial fibrillary acid protein (GFAP) and cytokeratin (CK). Based on these findings, a final diagnosis of Choroid meningioma (WHO GII) was made.
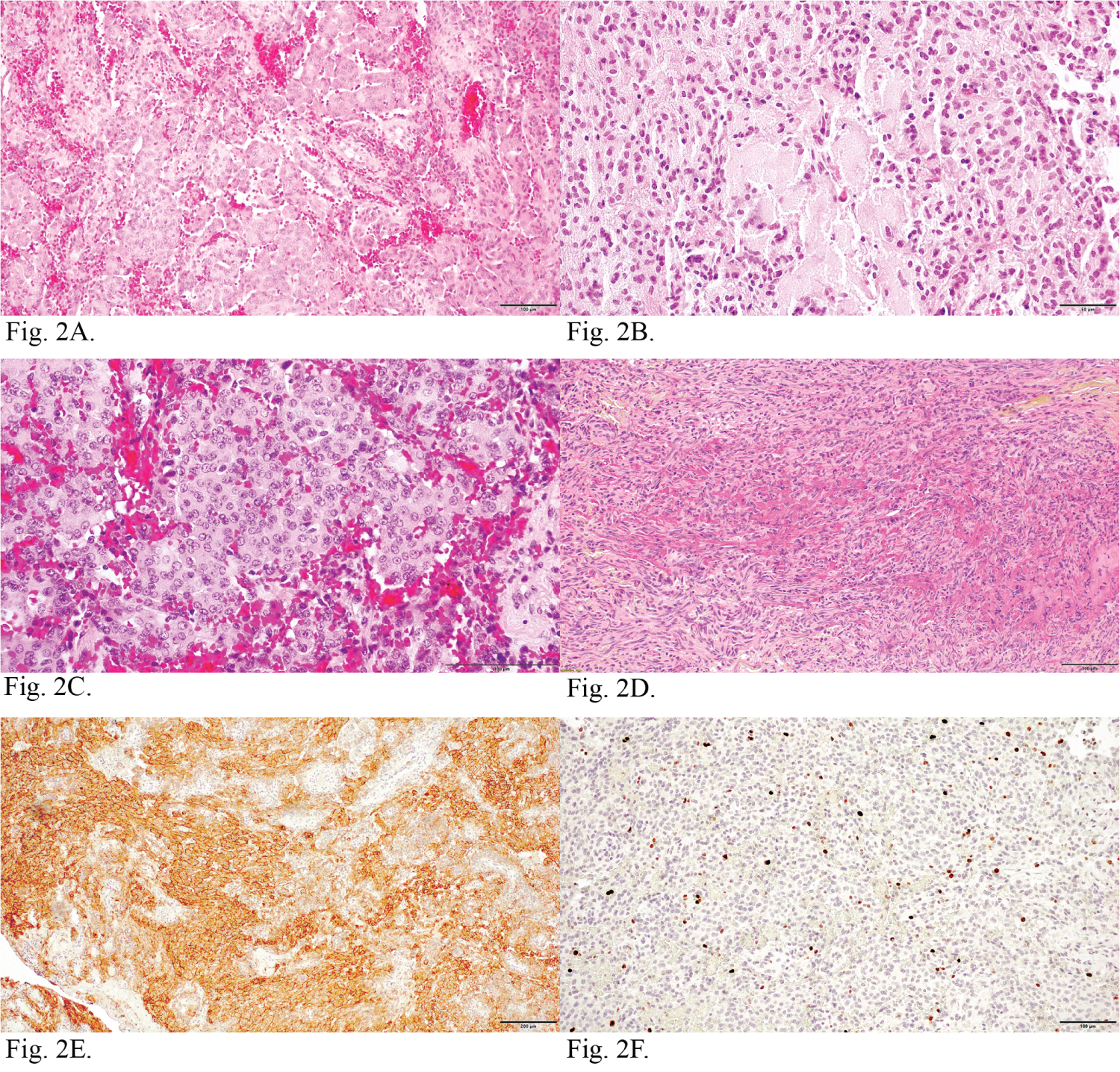 Figure 2: Neuropathological features of choroid meningioma with haemorrhage. (2A) Cords of tumor cells and trabeculae of round, ovoid and epithelial cells. H&E; (2B) The tumor cells were embedded in a prominent myxoid background. H&E; (2C) Typical meningothelial areas with haemorrhage. H&E; (2D) Tumor cells embeded in mucinous stroma. Mucicarmine; (2E) Cords of tumor cells with intense EMA immunoreactivity; (2F) Ki-67 labeling index shows nuclear immunoreactivity.
View Figure 2
Figure 2: Neuropathological features of choroid meningioma with haemorrhage. (2A) Cords of tumor cells and trabeculae of round, ovoid and epithelial cells. H&E; (2B) The tumor cells were embedded in a prominent myxoid background. H&E; (2C) Typical meningothelial areas with haemorrhage. H&E; (2D) Tumor cells embeded in mucinous stroma. Mucicarmine; (2E) Cords of tumor cells with intense EMA immunoreactivity; (2F) Ki-67 labeling index shows nuclear immunoreactivity.
View Figure 2
As the tumor removal was subtotal, patient age did not encouraged us to further resection, decision about complementary therapy was made in agreement with oncologist.
Chordoid meningiomas are very rare tumors, as they represent only 0.5% of all meningiomas. Less than 300 cases were reported worldwide up to the date. Kepes, et al. [7], who first described these lesions, reported their association with Castleman's syndrome. Couce, et al. [5] concluded that the systemic disease associated with chordoid meningiomas was restricted to children. No manifestation of systemic disease in adults was noted in his material.
Histologically, chordoid meningiomas are similar to chordomas. They contain trabeculae, or cords of eosino-philic vacuolated cells in a myxoid matrix [8]. High mitotic rate, increased cellularity, small cell change, sheet like growth, macro nucleoli and focal necrosis are present [3,9]. Loewenstern, et al. [9] noticed moreover, that the mitotic index is a direct indicator of cell proliferation and predictor of tumor behavior. Aghi, et al. [10] reported that prominent nucleoli were associated with higher risk of recurrence. In material of Lee, et al. [11] focal necrosis was related with six-fold risk of recurrence. Baltus, et al. [12] found, that detection of chro-mothripsis in chordoid meningioma can be related with considerable potential of recurrence after initial sur-gery. One of the most important regrowth patterns of the tumor is the high chordoid features percentage. It was first described by Couce, et al. [5]. Chordoid features greater than 50% as a significant tumor regression marker was emphasized also in later reports [13-16]. In the study of Matyja, et al. [17] significant chordoid component was concerned not only with high tumor recurrence rate, but also with more aggressive biological behavior. Hasegawa, et al. [8] described relation with high mucoid quality of the tumor with its rapid spread and recurrence.
As the extent of resection is the most important prognostic factor in case of chordoid meningioma treatment, Gross total resection (GTR) should be the goal for the surgical management of primary chordoid meningioma. It remains the strongest predictor of long-term rates of tumor control [4-9,15-20]. As the recurrence remains high following subtotal resection, careful follow up should be recommended in every case. The most illustra-tive reason for careful observation was presented by Couce, et al. [5]. In his long term follow up, all patients after a subtotal resection experienced tumor recurrence, while only 1 out of the 29 patients after gross total resections recurred [5]. Similar observations were noticed in the study of Zhu, et al. [21]. Although the most of authors recommend adjuvant radiotherapy for atypical meningiomas in the management of residual disease following subtotal resection [14,22,23], its efficacy remains unclear. In cases of incomplete resections, Liu, et al. [24] justified its application as a method of salvage treatment. Conversely, in cases of complete tumor re-sections, most of the authors found no significant progression free survival rates increase after radiotherapy [4,13,21-24]. Only few case studies advocated for the role of radiotherapy after Gross total resection [25,26].
The MIB-LI (cell proliferation marker) is a histological marker for proliferative capacity, correlated with high rates of tumor control [4]. Higher MIB-LI has been noted to correlate with recurrent meningiomas in studies of some authors [27-30]. Others did not share this opinion, founding no correlation with outcomes [13,30,31].
Other factors concerned with unfavorable overall results were presented by Zhang, et al. [32]. In this large retrospective analysis, male gender, larger tumor size, surgical history and bone invasion were defined as risk factors associated with poor outcome.
Choroid meningiomas constitute a rare and diverse subtype of WHO grade II meningiomas. The review of the literature evidenced, that the most important factor leading to progression free survival is gross total resec-tion. The role of adjuvant radiotherapy remains controversial. It should be indicated in patients with subtotal resection and high MIB-LI.