A giant cell granuloma (GCG) rarely occurs in the skull bones [1]. Although it has destructive character, the GCG is used as the synonym of giant cell reparative granuloma (GCRG) in the literature. Differentiation from other osteolytic lesions quite difficult. The first case of GCG in the temporal bone was reported by Hirschl and Katz in 1974 [2]. Even though the GCG is not a true neoplasm, the locally aggressive behavior of this tumor necessitates surgical excision whenever possible.
A 36-year-old male healthy patient admitted with the complaint of pain, and swelling around left the temporomandibular region. Neurologic examination revealed hypoaesthesia in the maxillary and mandibular division of Vth nerve. Routine blood examination was normal including serum and urinary level of Ca, P, alkaline phosphatase and parathyroid hormone. Neurologic examination revealed hypoaesthesia in the maxillary and mandibular division of Vth nerve. Cranial computed tomography (CT) showed a bulky heterogeneous mass sized in 40 × 30 × 35 mm which caused expansion and destruction of spongious bone at basal and squamous part of the temporal bone (Figure 1). The mass enhanced nonhomogeneously after contrast media injection. The magnetic resonance imaging (MRI) showed heterogeneous, multiloculated extra-axial lesion, and after gadolinium injection peripheral enhancement was seen on T2W images (Figure 2). Diffusion-weighted image (DWI) showed no diffusion restriction. The patient embolized the day before the operation to decrease its vascular supply. At operation, the left question mark incision encircling the lesion was performed. An enormous compact mass, which had soft and firm points on it, appeared after the elevation of temporal muscle on temporal bone. A burr hole was performed on the normal appearing squamosal temporal bone, and linear craniectomy was performed within normal squamosal bone around the mass. Dura mater dissected easily from the mass, and there was no dural involvement. Its medial border reached to the lateral edge of the foramen rotundum and ovale. The mass excised totally and fairly bloodless due to embolization of the tumor. The bone defect was repaired with titanium mesh.
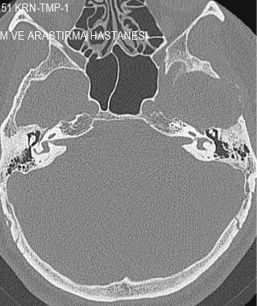 Figure 1: Squamous part destruction of the left temporal bone. View Figure 1
Figure 1: Squamous part destruction of the left temporal bone. View Figure 1
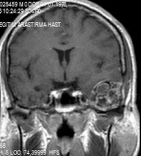 Figure 2: T1W contrast-enhanced image showed heterogeneously enhanced extra-axial lesion after gadolinium. View Figure 2
Figure 2: T1W contrast-enhanced image showed heterogeneously enhanced extra-axial lesion after gadolinium. View Figure 2
In the microscopic examination, there are mononuclear epitheloid fibrohistiocytic cells, macrophages with, hemosiderine, extravasated erythrocytes and multinuclear giant cells. Multinuclear giant cells are non-homogen, dispersed around the stroma. Mononuclear cells are positive with CD 68, negative with S-100, CD1a and P63. Mitotic acitvity is rare. Also, there are giant cells around thin-walled cystic space (Figure 3). The Ki-67 index was 2%. The lesion was reported as GCG. After 5 years of operation, there was no recurrence.
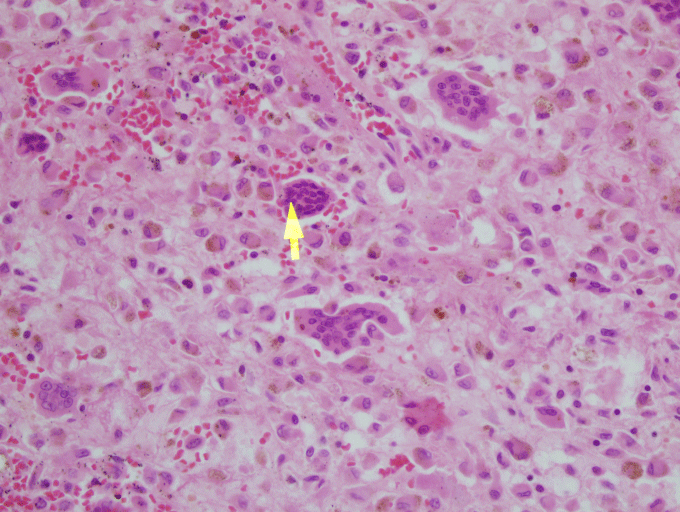 Figure 3: x40, HE, microscopic examination showed multinuclear giant cells are dispersed around the stroma (arrow indicates multinuclear giant cell). View Figure 3
Figure 3: x40, HE, microscopic examination showed multinuclear giant cells are dispersed around the stroma (arrow indicates multinuclear giant cell). View Figure 3
The pathogenesis of GCG is unknown. Jaffe [3] considered the lesion as a cause of reparative process due to intraosseous hemorrhage or periosteal reaction due to trauma. But many cases reported in the literature have no history of trauma as in our patient. Other theories include infectious and developmental causes [2,4]. The origin of multinucleated giant cells are speculative. It is alleged in the literature that multinucleated giant cells (MGC) originate from phagocytic MGC, osteoclastic lineage, endothelial cell, fusion of stromal mononuclear cells [5]. Local trauma or irritation induces proliferation of fibroblasts, secretion of collagen, desmoplastic changes in parenchymal and stromal tissues. All of these events cause transformation of fibroblastic cells to a myofibroblastic phenotype. In cytogenetic studies, different chromosomal abberations are also seen in GCT (16q22, 17p13) and GCG (X, 4q22, q31.3) [5].
Pathologic differentiation between GCG and giant cell tumor (GCT) is possible with microscopic examination. The GCG contains more fibrous stroma, hemosiderin deposits, infiltrative lymphocytes, and reactive bone formation compared with GCT. The giant cells are located focally in GCG and contain fewer nuclei than those of GCT [2]. MGCs of extracranial GCT and GCG are immunohistologically positive for CD 68, and shows histiocytic differentiation [6]. We also detected CD68 positive cells in the presented case, and P63 immunostaining may use differentiating GCTs from GCGs, and it is positive in GCT [7]. Negative P63 immunostaining indicated that the cells were not of epithelial origin. The Ki-67 index was 2%.
The incidence is highest in the second decade of life [8]. The GCG most often appears as a slowly growing mass. Mass effect, otalgia, a headache, hearing loss, and cranial nerve palsies (56%) are the most common presenting symptoms [7,8]. The presented patient applied with swelling and pain in the left temporomandibular area. He also had hypoesthesia at V2, and V3 distribution of the Vth nerve because of the near vicinity of the foramen rotundum and ovale.
The GCGs appear as an osteolytic lesion on skull radiography and has marginal sclerosis on CT [7]. Nackos, et al. [8] published CT findings of 7 cases GCG, in which all 7 cases showed mixed density, and enhanced heterogeneously. There was an expansion with the remodeling of the adjacent bone and lytic bone destruction as well as intralesional mineralization on CT [8]. We detected similar CT appearance in our patient with marginal sclerosis and solid-cystic components indicated remodeling and destruction (Figure 1). MRI of GCGs show heterogenous intensity on both T1 and T2W images [6]. The presented patient also had mixed density in both T1 and T2W images, and contrast enhancement was heterogenous after contrast media injection (Figure 2). DWI is a valuable method for differentiation of a bone lesion that it is benign or malign [9]. The low signal on DWI was detected on MRI in the presented case was thought the lesion was benign nature.
The GCG consists of non-neoplastic vascular tissue, and angiography shows hypervascularity due to feeding vessels arising from the external carotid artery [10]. Preoperative angiography and feeding vessel occlusion were performed in that case the day before the operation, and the mass was excised without needing the blood transfusion.
The differential diagnosis must be made between an aneurysmal bone cyst, Brown tumor of hyperparathyroidism, fibrous dysplasia, chordoma, chondrosarcoma, and intradiploic meningioma. Fibrous dysplasia, aneurysmal bone cyst, and Brown tumor of hyperparathyroidism also have marginal sclerosis and soap bubble appearance in radiologic examinations. Differentiation between the Brown Tumor and other pathologies is based on patient's parathyroid hormone level, serum and urinary calcium, and alkaline phosphatase levels. The single lesion and negative blood tests eliminated the Brown tumor in presented case. The radiologic differentiation between GCT and GCG is not possible. The presence of calcifications on CT tends to lead more toward the diagnosis of chordoma, chondrosarcoma, and intradiploic meningioma.
The GCG is not a true neoplasm. Although the reported cases indicate that the biological behavior of GCG involving the skull is benign, the preferred treatment is complete surgical excision with negative tumor margin [6]. But this is not possible all time due to the involvement of skull base bones. Preoperative embolization of the mass before surgical excision is very helpful for excision with negative tumor margin as in our case due to the bloodlees surgical field and allows for the complete excision of the tumor. Spontaneous remission is also reported [11]. Ten to fifteen percent of cases of GCG recurs after inadequate removal [2]. In recurrent cases, attempting total surgical excision must be thought. Although radiotherapy is another option for recurrent tumors, malignant degeneration has been reported after radiotherapy in 7-29% of the cases and had no proved effect on local control of the tumor [6].
New treatment methods such as subcutaneous injection of osteoclastic inhibitors alone or combined with corticosteroid or subcutaneous calcitonin injection as a parathormone antagonist or alpha interferon as an antiangiogenics agent have also some success.
No any funding.