Obstructive sleep apnea (OSA) is a sleep respiratory disorder characterized by an intermittent complete or partial collapse of the upper airways, resulting in apnea and hypopnea events. Breathing pauses cause acute adverse effects including oxyhemoglobin desaturation, changes in blood pressure and heart rate, increased sympathetic activity, cortical arousals (micro awakenings) and sleep fragmentation [1].
There is limited evidence to support the fact that mild OSA can have negative health consequences, but numerous studies on large populations have shown the correlation between moderate or severe untreated OSA with various complications [2].
It was shown, after controlling for confounding factors such as body mass index and smoking, that severe OSA confers a 2.6 times greater risk of myocardial infarction, coronary revascularization, congestive heart failure or cardiovascular death [3]. The risk of ischemic stroke is also increased in patients with untreated OSA, particularly in men with an apnea-hypopnea index (AHI) greater than 19 events/hour or in women with an AHI of more than 25 events/hour [4]. Other important cardio-metabolic associations include atrial fibrillation, resistant hypertension and insulin resistance [5,6].
Although the causality between OSA and arrhythmia has not yet been established, the emerging data have identified a number of pathophysiological pathways that can increase the propensity for cardiac arrhythmia in OSA. These pathways are complex, multidirectional and potentially synergistic. OSA and atrial fibrillation, for example, share risk factors (increasing age, obesity, hypertension) and consequences that can act together in increasing cardiovascular risk [7]. Epidemiological studies have shown that sleep breathing disorders carry an almost double risk of atrial fibrillation; in patients with OSA and heart failure, the risk increases 2 to 4 times compared to those without either condition [8-17]. Although most studies have focused on the links between OSA and atrial fibrillation, relationships with ventricular arrhythmias have also been described [18-20]. Furthermore, studies on circadian rhythm and sudden cardiac death have shown a predisposition in sleep apnea subjects to develop fatal events during the night, a period of relative cardioprotection for individuals without obstructive apnea [21]. Bradyarrhythmias, such as atrioventricular blocks and sinus pauses, are also more prevalent in patients with OSA, however epidemiological studies have shown that the association is limited to those with severe obstructive apnea (AHI ≥ 30 events/hour) and with severe nocturnal hypoxia; in particular, atrioventricular nodal blocks have been observed mainly in REM sleep, when apneas are typically longer and oxygen desaturation is more pronounced [22].
The apneas and hypopneas that characterize OSA are frequently accompanied by oxyhemoglobin desaturations and microarousal, which cause sleep fragmentation. The imbalance of the autonomic nervous system, characterized by vagal cardiac activation during and by an undisputed sympathetic activation after OSA events, can precipitate arrhythmias [23,24]. In addition, important fluctuations in intrathoracic pressure occur in OSA caused by attempts to breathe in the presence of blocked airways [25]. These pathophysiological events related to obstructive sleep apnea can lead to an increase in the arrhythmia rates observed in patients with these disorders [9,26]. Overall, in OSA it is possible to consider a conceptual model based on repeated acute insults (i.e. continuous hemodynamic, hypoxemic and autonomic variations) that lead, over time, to a structural and electrical cardiac remodeling capable of constituting a potentially arrhythmogenic substrate (Figure 1).
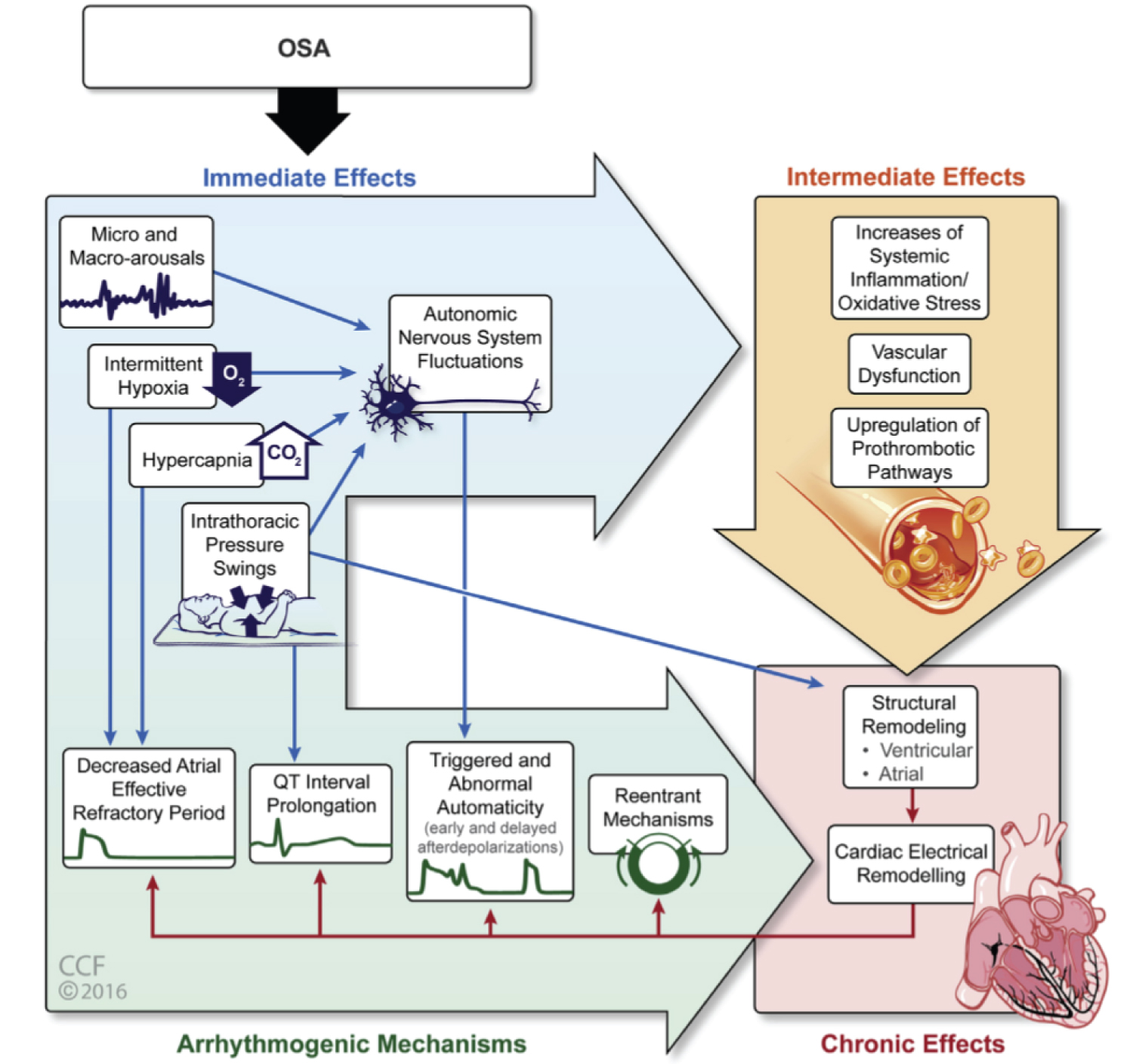 Figure 1: Pathophysiological pathways of obstructive sleep apnea that potentially predispose to cardiac arrhythmogenesis (Cleveland Clinic Center for Medical Art & Photography© 2016).
View Figure 1
Figure 1: Pathophysiological pathways of obstructive sleep apnea that potentially predispose to cardiac arrhythmogenesis (Cleveland Clinic Center for Medical Art & Photography© 2016).
View Figure 1
The electrophysiological bases in OSA-induced cardiac arrhythmia include abnormal automatism, triggered activity, reduction of the atrial effective refractory period (ERP), QT interval prolongation and reentry mechanisms [7].
Although there are unambiguous synergies between the pathophysiological sequelae of OSA, the data suggest that OSA-related sympatho-vagal imbalance may be the main trigger for cardiac arrhythmogenesis.
The progressive increase in respiratory effort to obtain the restoration of the patency of the airways is intrinsic to the physiology of obstructive respiratory events. During the apnoic event, vagal stimulation to the heart increases, leading to bradycardia (immersion reflex). These vagal influences shorten the atrial effective refractory period and thus increase vulnerability to excitatory stimuli [7]. When upper airway patency is restored, strong sympathetic nervous system responses are elicited due to the respiratory center-sympathetic interaction, the synergistic effects of hypoxia and hypercapnia, and the concomitant lack of sympathetic inhibition from normal pulmonary reflexes [27].
Furthermore, the increase in sympathetic nervous activity in OSA is largely responsible for the characteristic heart rate and blood pressure peaks that generally occur shortly after the end of apnea [28-30]. These repetitive increases in blood pressure oppose the physiological reduction in values that accompanies normal sleep and can be responsible, in many cases, for the phenomenon of the "non-dipping" of the nocturnal pressure profile [31].
However, the autonomic imbalances associated with OSA are not limited to sleep. Patients with OSA have a higher diurnal sympathetic nervous activity than healthy controls matched for age, sex and body mass index [32,33].
Treatment of OSA with continuous positive airway pressure leads to a reduction in urinary catecholamine levels at night and in daytime muscular sympathetic nervous activity. The latter effect occurs after several months of CPAP therapy and is most evident in patients with the most hours of CPAP use [34-36].
Cardiovascular autonomic function in OSA was also examined by evaluating heart rate variability (HRV) [27]. HRV is the variation of the time intervals between adjacent heart beats and is considered a measure of neurocardiac function that reflects, in fact, heart-brain interactions and the dynamics of the autonomic nervous system. A healthy heart is not a metronome and the oscillations between beats are complex and non-linear [37]. An optimal HRV value generally reflects a good state of health, the psycho-physical adaptability and resilience of the individual [38] while reduced heart rate variability is associated with an increased risk of cardiac events and mortality [39]. A common cause of HRV reduction is persistent activation of the sympathetic nervous system [40].
Some studies have shown that OSA is able to influence HRV by causing an increase in sympathetic modulation and a reduction in the parasympathetic one [27].
The objective of our study was to examine the potential effects of obstructive sleep apnea on arrhythmias and autonomic cardiac activity by evaluating, respectively, the rate of both supraventricular and ventricular extrasystoles and heart rate variability indices (HRV) in subjects with OSA compared to a control group.
From 2015 to 2019, about 1160 patients accessed the clinic for sleep disorders, belonging to the UOC of Geriatrics of the Policlinico Umberto I in Rome, to perform nocturnal cardiorespiratory monitoring or polygraphy.
Of this sample we enrolled 65 patients who, in addition to having performed the polygraphic survey, were also subjected to a 24-hour dynamic electrocardiogram for clinical study.
Polygraphy found an AHI ≥ 5 events/h in 55 patients, who were therefore diagnosed with OSA, while it was negative (AHI < 5 events/h) in the remaining 10 patients, who were classified as controls.
All patients were asked for complete medical history, including drug therapy in progress, and questionnaires were given to investigate daytime sleepiness, snoring, and sleep quality (Epworth Sleepiness Scale, Berlin Test, Pittsburgh Sleep Quality Index). In addition, in each of them, anthropometric parameters such as weight, height, body mass index (BMI), waist and neck circumference were measured.
The polygraphic recording was performed using the "Embletta MPR" or "Nox T3" equipment. Signals detected by the devices included peripheral oxygen saturation (SpO2), nasal airflow, chest and abdominal movements, heart rate, snoring, and body position. All overnight cardiorespiratory monitors had a valid recording time of at least 4 hours.
The data were interpreted by integrating the automatic analysis of the "RemLogic" or "Noxturnal" software with the manual analysis of the medical staff. Apnea was defined as the absence or reduction in nasal airflow ≥ 90% for a period of ≥ 10 seconds while hypopnea as the reduction in airflow ≥ 30% for a period of at least 10 seconds associated with a oxyhemoglobin desaturation ≥ 3%.
The AHI was derived from these respiratory events, which is the total number of apneas and hypopneas per hour of sleep. The ODI was defined as the number of oxyhemoglobin desaturation episodes ≥ 3% compared to baseline per hour of sleep.
All enrolled patients underwent 24-hour electrocardiogram monitoring using the 3-channel "North East Monitoring DR200" device. The recorded data were analyzed using the "LX Analysis 5.4c" software and the tracings were fully reviewed by the medical staff for the correction and elimination of any artifacts.
For each patient, the total number of supraventricular (SVES) and ventricular extrasystoles (VES) was measured, as well as the maximum, minimum and average heart rate values.
Regarding supraventricular extrasystoles, they were defined as frequent when present in numbers greater than 76 per day, referring to a study published in the "Journal of the American Heart Association" in 2015 entitled "Prognostic Significance of Premature Atrial Complexes Burden in Prediction of Long- Term Outcome" [41]; while ventricular extrasystoles were considered frequent if higher than 30 per hour (720 per day) on the basis of the "Lown classification" [42].
From the 24-hour electrocardiographic recording, using the same software "LX Analysis 5.4c", the variability of the heart rate was evaluated, i.e. the variation of the R-R interval between adjacent heart beats, both in the time domain and in that of the frequency.
The time domain indices, obtained through statistical operations on the R-R interval, include: the standard deviation of all R-R intervals (SDNN); the square root of the mean square differences of the adjacent R-R intervals (RMSSD); the percentage of adjacent R-R intervals that differ from each other by more than 50 ms (pNN50).
In the frequency domain, instead, through the spectral analysis, the following HRV variables were evaluated: Very low frequency (VLF), low frequency (LF), high frequency (HF), total power (TOTAL), low frequency/high frequency (LF/HF).
The characteristics of the HRV indices are summarized in Table 1.
Table 1: The definitions of heart rate variability parameters. View Table 1
Normal continuous variables were expressed as mean ± standard deviation while non-normal continuous variables were expressed as median [interquartile range - IQR]. The statistical tests used were, when appropriate, Student's t and Mann-Withney respectively. The categorical variables were expressed in percentage values and the significance of the differences tested with the chi-square test.
A multivariate logistic regression model was conducted to test the variables associated with the presence of a significant number of SVES and/or VES. A value of p < 0.05 was accepted as statistically significant. Statistical analysis was conducted using IBM SPSS Statistics 25.0 software.
The clinical characteristics compared between the OSA patients and the control group are summarized in Table 2.
Table 2: Main clinical features in patients with OSA and in control subjects. View Table 2
The age of the patients did not appear very divergent in the two groups, while the BMI, waist and neck circumference were significantly higher in those with OSA (p < 0.01). The prevalence of some diseases such as hypertension and diabetes mellitus evidently appeared higher in patients with obstructive apnea (p < 0.05) as well as dyslipidemia, the latter, however, without statistical significance. Furthermore, the frequency of ventricular extrasystoles over 24 hours was also significantly higher in OSA subjects compared to controls (p < 0.01); the same trend was observed for supraventricular extrasystoles without however reaching the statistical weight.
By analyzing more in depth the potential impact of OSA on cardiac arrhythmias, the prevalence of excessive frequency of supraventricular and/or ventricular extrasystoles per day was assessed (defined > 76/24 h for SVES and > 720/24 h for VES in reference the above studies) compared to the presence or absence of sleep breathing disorder. The difference between groups was statistically significant (p = 0.044) and as can be seen from Chart 1, in the presence of OSA the number of patients with an excessive number of supraventricular and/or ventricular extrasystoles per day increases.
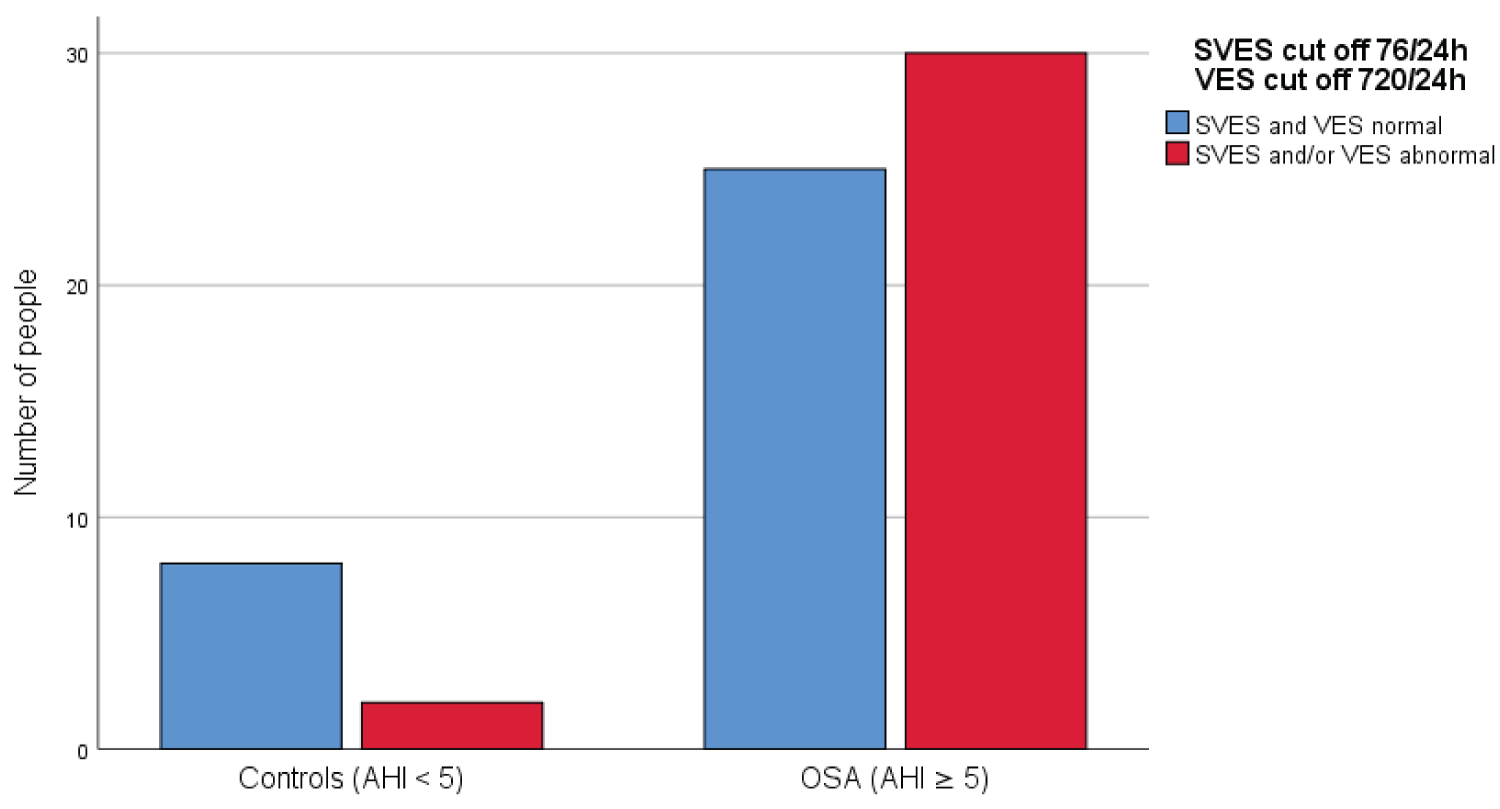 Chart 1: Prevalence of the excessive number of extrasystoles in OSA patients and controls.
View Chart 1
Chart 1: Prevalence of the excessive number of extrasystoles in OSA patients and controls.
View Chart 1
In addition, the different prevalence of the increased number of extrasystoles in the different classes of OSA was evaluated; also in this case the statistical significance was highlighted (p = 0.000) and by examining Chart 2, it is possible to note that as the severity of OSA increases, the number of patients with extrasystoles above the threshold increases; this trend, however, was observed in mild and moderate degrees of disease but not in severe OSA, in which a reduction in patients with an altered number of extrasystoles was highlighted.
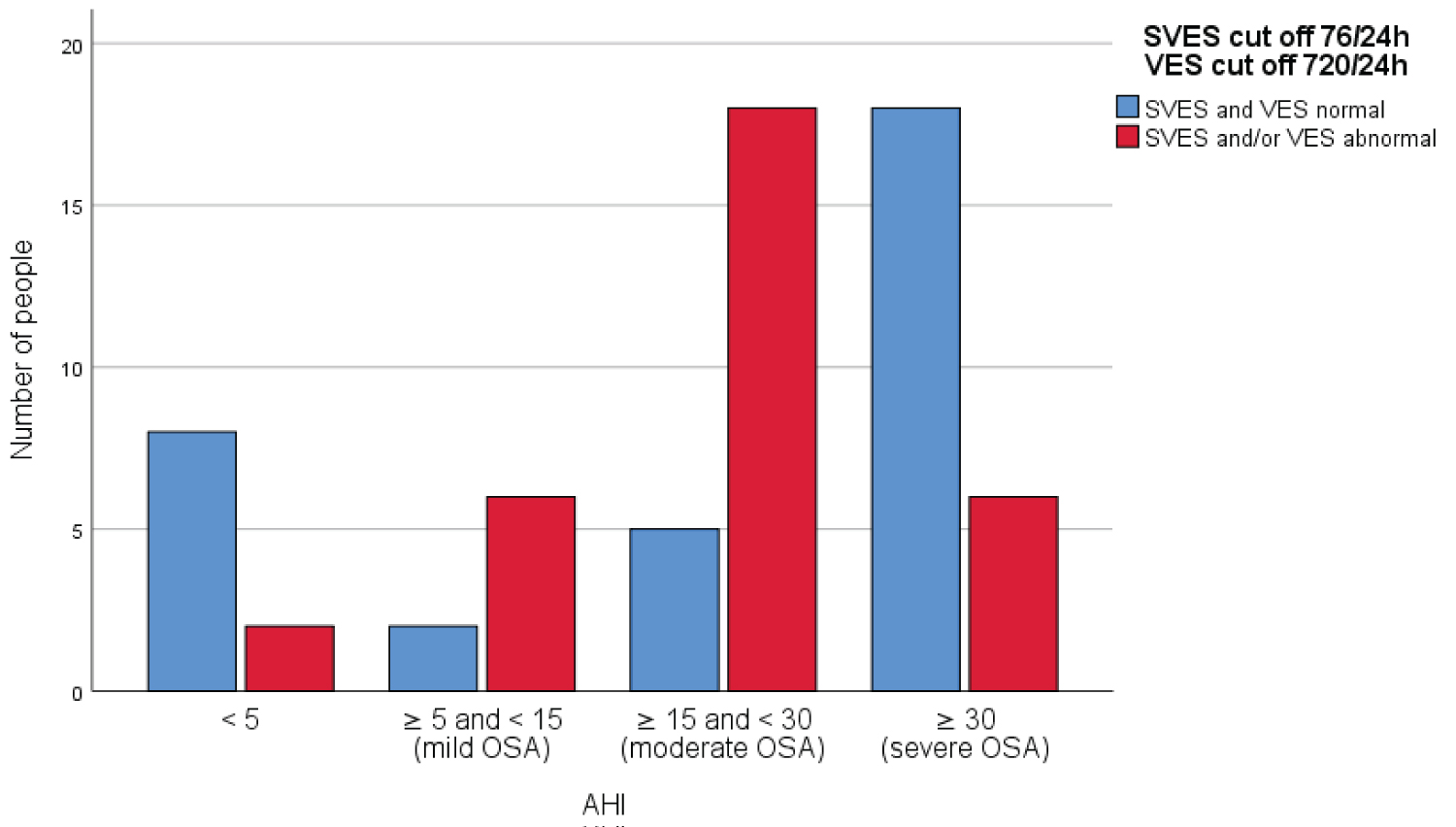 Chart 2: Prevalence of the excessive number of extrasystoles in the various degrees of OSA.
View Chart 2
Chart 2: Prevalence of the excessive number of extrasystoles in the various degrees of OSA.
View Chart 2
In the hypothesis of a possible influence of antiarrhythmic therapy, the frequency of the latter was analyzed compared to the presence or absence of severe OSA and a statistically significant difference emerged; as shown in Chart 3, in fact, it was found that the intake of antiarrhythmic drugs is more frequent in patients with severe obstructive apnea than in those with lower degrees of disease (p = 0.015).
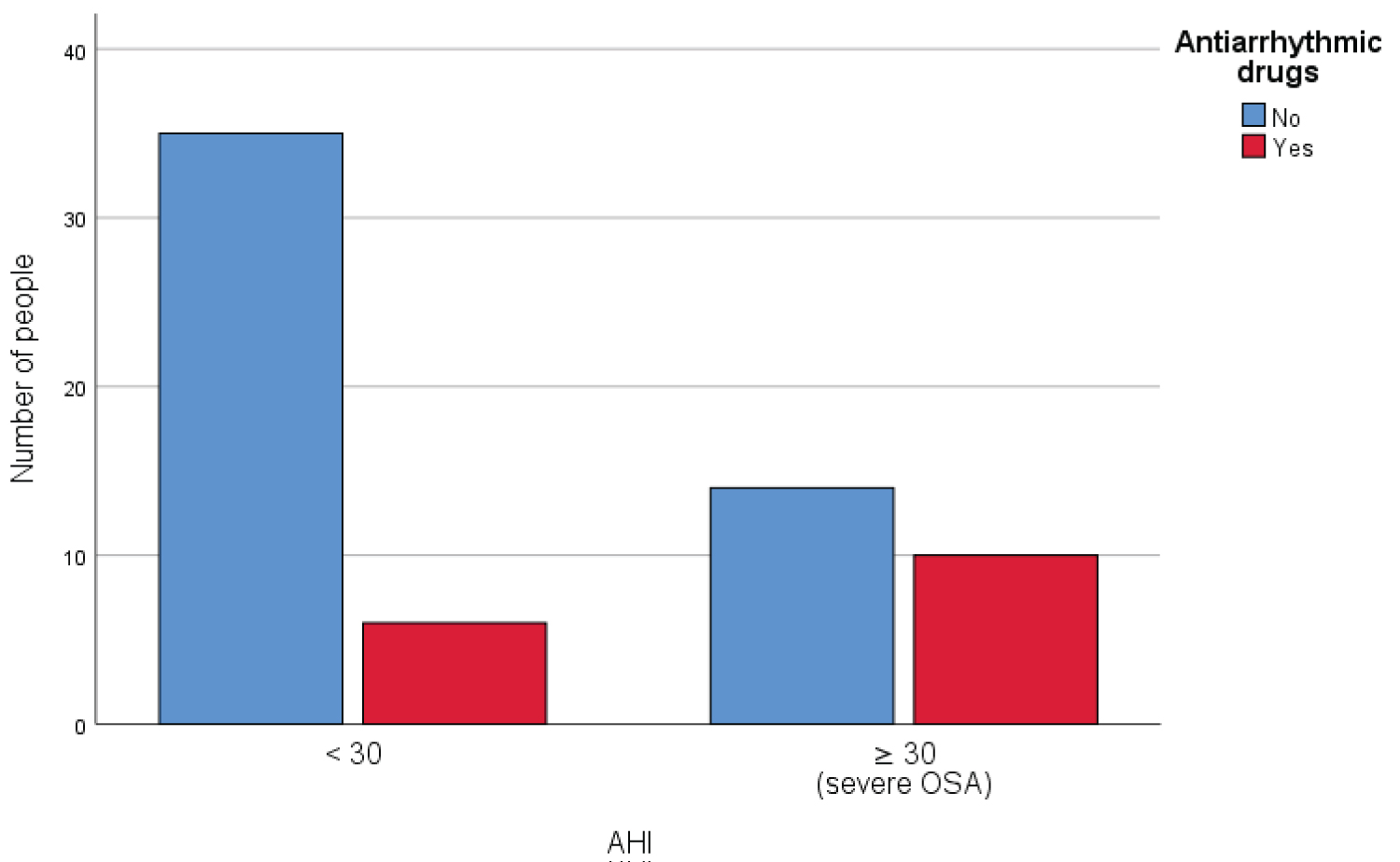 Chart 3: Prevalence of the antiarrhythmic therapy in patients with severe and non-severe OSA.
View Chart 3
Chart 3: Prevalence of the antiarrhythmic therapy in patients with severe and non-severe OSA.
View Chart 3
Ultimately, through a multivariate logistic regression model it was found that OSA is associated with the increase in extrasystoles above the threshold value regardless of gender, the age of over 65 years, the BMI class, the presence of pathologies such as arterial hypertension and diabetes, and the antiarrhythmic therapy (Table 3).
Table 3: Logistic regression. View Table 3
In the study, HRV indices and mean heart rate values were also compared between the OSA group and controls (Table 4), but excluding patients on antiarrhythmic therapy from the analysis to avoid confounding factors deriving from the effects of the same on the heart activity.
Table 4: The differences of HRV parameters and average heart rate between OSA patients and controls. View Table 4
From the comparison survey between the two groups, only one statistically significant difference emerged regarding the SDNN value, which was lower in patients with OSA.
In addition, the analysis was performed to compare the HRV and average heart rate values between patients with severe OSA, therefore overt, and controls (Table 5).
Table 5: The differences of HRV parameters and average heart rate between patients with severe OSA and controls. View Table 5
As can be seen from Table 5, in severe OSA compared to the control group, the SDNN value was statistically significantly decreased while the average heart rate was found to be higher.
Obstructive sleep apnea is a very common disease and is associated with an increased risk of cardiovascular events such as myocardial infarction, atrial fibrillation, sudden cardiac death and other arrhythmias. The correlation between heart rhythm disorders and OSA is an emerging problem and is increasingly being studied [43]. Various pathophysiological consequences of OSA, including intermittent hypoxia and the resulting oxidative stress, recurrent arousals and fluctuations in intrathoracic pressure can cause cardiac arrhythmias, both directly and through the effects on the autonomic nervous system [22].
In this study, in agreement with previous evidence [18,20] it was found that patients with OSA have a significantly higher number of ventricular extrasystoles per day than healthy patients. In addition, in the presence of the disease, the number of patients with an excessive daily frequency of supraventricular and/or ventricular extrasystoles increases. Finally, OSA was associated with an increase in extrasystoles above the threshold value set regardless of gender, age over 65, BMI class, the presence of diseases such ashypertension and diabetes, and taking antiarrhythmic drugs.
In an analysis that evaluated the impact of ventricular ectopic beats on cardiac function, it was found that an increased rate of ventricular extrasystoles detected on 24-hour electrocardiographic monitoring is associated, over time, with a decrease in left ventricular ejection fraction, an increase in the incidence of congestive heart failure and an increase in the mortality rate [44].
Other authors who have analyzed instead the prognostic significance of an excessive number of supraventricular extrasystoles have suggested that a high frequency of atrial premature beats, in the long term, is independently associated with mortality, hospitalization for cardiovascular events, the onset of atrial fibrillation and pacemaker implantation [41].
However, it has been shown that in patients with OSA, CPAP treatment, in some cases, can reduce the frequency of cardiac arrhythmias [45].
As already discussed, one of the main factors contributing to the arrhythmic propensity in OSA is the continuous fluctuation of the autonomic nervous system, characterized by an enhancement of parasympathetic activation during and by sympathetic waves after obstructive respiratory events. The cardiovascular system is therefore chronically exposed to a stress that is repeated many times every night, characterized by large and repeated oscillations in blood pressure and heart rate [46]. Furthermore, the continuous stresses caused by nocturnal obstructive respiratory events, over time, can lead to an autonomic imbalance. However, the anomalies in the autonomic regulation of the cardiovascular system associated with OSA are not only present during sleep but can also persist during the waking hours [27].
The measurement and analysis of heart rate variability (HRV) is a non-invasive method of evaluating autonomic heart function. HRV is the variation of the time intervals between adjacent heart beats; it reflects the combined activity of sympathetic and parasympathetic tone on heart rate and acts as a measurable indicator of cardiovascular health and prognosis [47]. Heart rate variability can be analyzed in the time domain and the frequency domain.
Time domain measurements are simple statistics derived from beat-to-beat intervals of sinus origin and are expressed in time units (ms). The time domain indices analyzed in this study include: the standard deviation of all R-R intervals (SDNN); the square root of the mean square differences of the adjacent R-R intervals (RMSSD); the percentage of adjacent R-R intervals that differ from each other by more than 50 ms (pNN50).
The frequency domain approach, instead, is based on the identification and quantification (in terms of frequency and power) of the main oscillatory rhythms of physiological origin that make up a sequence of RR intervals [48]. In the frequency domain, the following HRV variables were evaluated in this study: very low frequency (VLF), low frequency (LF), high frequency (HF), total power (TOTAL), low frequency/high frequency (LF/HF). The HF component reflects the activity of the parasympathetic nervous system, while the LF component is generally associated with sympathetic activity. However, the etiology of LF remains questionable as some studies suggest it is primarily under sympathetic control while others argue that it is the result of sympathetic and parasympathetic influences. Finally, the LF/HF ratio acts as an index to evaluate the relationship between sympathetic and parasympathetic activity (vagal tone), but it appears to be markedly influenced by various factors such as stress, rate and depth of breath [47].
In our survey, in agreement with other studies in the literature [49,50], the comparison analysis of the HRV indices between the OSA group and that of the controls showed a statistically significant difference in the SDNN value, which was lower in patients with obstructive apnea; moreover, this parameter was similarly reduced in patients with severe OSA when compared with controls, associated, in this case, also with a significant increase in the average heart rate value, likely expression of a prevalence of activity sympathetic nervous system over the parasympathetic one.
From previous evidence, the SDNN parameter has been shown to have a predictive value for mortality [37]. Kleiger, et al. [51], in fact, demonstrated that, following a myocardial infarction, the reduction of the SDNN, the ectopic ventricular activity and the reduction of left ventricular ejection fraction are associated with an increase in the mortality rate. Similar results were provided by another study, in which sympathetic-parasympathetic imbalance, expressed by reduced HRV (SDNN < 70 ms), was found to be a significant predictor of total cardiac mortality [52].
In conclusion, the data emerging from our study converge with those reported in the literature in confirming the presence of an increased risk of cardiac arrhythmias in OSA.
Obstructive sleep apneas, through pathophysiological mechanisms still not fully understood, are capable of causing alterations in autonomic cardiac activity and these could be implicated in the genesis and/or progression of associated cardiovascular diseases.
The HRV analysis represents a valid and non-invasive method for detecting such changes and it is conceivable that it can be used for the screening of OSA which, to date, is a very widespread but still largely underdiagnosed pathology.