The present review is based on a critical literature revision and aimed to identify the overall clinical behavior of laryngeal solitary fibrous tumor (SFT). Laryngeal hemangiopericytomas (HPC) were included, according to both their re-classification as the SFT's cellular variant and the NAB2-STAT6 fusion gene. A case series was builded including a new case and other 30 previously published cases of laryngeal SFTs (Medline 1956 to 2021) with comparative purpose. Immunohistochemistry and molecular analysis were performed on the new case. The clinical features of such case series showed a peculiar behavior of SFT, characterized by 1) Early symptomatic onset 2) Small size at diagnosis 3) Prevalent supra-glottic location 4) Impairment of the quality of voice with vocal muscle sparing 5) Favorable clinical outcome after complete surgical excision, since no distant metastases were reported in the literature. Furthermore, atypical histologic features were infrequently described. As seen in the pleural counterpart and in other extra-pleural locations, laryngeal SFT harboured NAB2-STAT6 gene fusion and showed a strong nuclear immunostaining for STAT6, as confirmed by the herein described case. According to its peculiar clinical-pathologic behavior, the risk stratification models that have been proposed for SFT are not suitable for the larynx.
Solitary fibrous tumor, Hemangiopericytoma, Larynx, Malignant transformation, NAB2-STAT-6 fusion gene
Solitary fibrous tumor (SFT) is an uncommon mesenchymal neoplasm characterized by fibroblastic differentiation, first described in the pleura [1] and virtually affecting all body sites. It belongs to a spectrum of mesenchymal tumors, including hemangiopericytoma (HPC) [2,3]. In the head and neck region, SFT has been described in the oral cavity [4], ear [5], nasal cavity and para-nasal sinuses [6], orbit [7], parotid gland, and soft tissue [8], whereas it is rare in the larynx. Both clinical and radiologic findings are non-specific, making the histological assessment key in the diagnosis, based on architectural, cytomorphological, and immunohistochemical findings [8,9]. A recurrent intra-chromosomal paracentric inversion involving the long arm of chromosome 12, resulting in NAB2-STAT6 gene fusion, has been recently detected in both HPC and SFT [10,11]. Extra-pleural SFT can display atypical features and malignant transformation, indolent course, and unpredictable biological behavior [2,3]. In this regard, several risk assessment models have been recently proposed [12,13].
Herein we describe a new case of laryngeal SFT. A 79-year-old non-smoking woman presented with progressive hoarseness and dyspnoea. She denied dysphagia, weight loss, and otalgia. A fiber-optic laryngoscopy revealed a firm submucosal mass involving the left aryepiglottis fold. Vocal mobility was preserved on both sides. MRI imaging showed a well-defined T1 and T2 hypo-intense supraglottic mass, heterogeneously enhancing with contrast, dislocating the adjacent structures. Subsequently, a CO2 laser excision was performed, allowing a complete tumor resection. The surgical removal was curative, providing airflow improvement and subsequent symptomatic relief. The clinical course was uneventful, with no evidence of disease after 5 years-long follow-up. Grossly, the tumor appeared as well demarcated uncapsulated mass with grey-to-yellow cut surface, measuring 41 × 32 × 24 millimeters (Figure 1). The whole tumor was processed for histopathological evaluation. Specifically, 4 μm-thick sections were obtained from formalin-fixed paraffin-embedded blocks and stained with Hematoxylin-Eosin. The histologic assessment showed a hypercellular proliferation of spindle cells with a haphazard growth pattern, overriding rare hypocellular areas. Pleomorphic and crowded nuclei with vesicular chromatin were seen. Mitotic count was performed on 10 high power field (HPF), both in the hypercellular and hypocellular areas. Such features were consistent with a low-grade tumor with fibroblastic differentiation reminiscent of HPC, according to the previous classification. The tumor margins showed a pushing growth pattern.
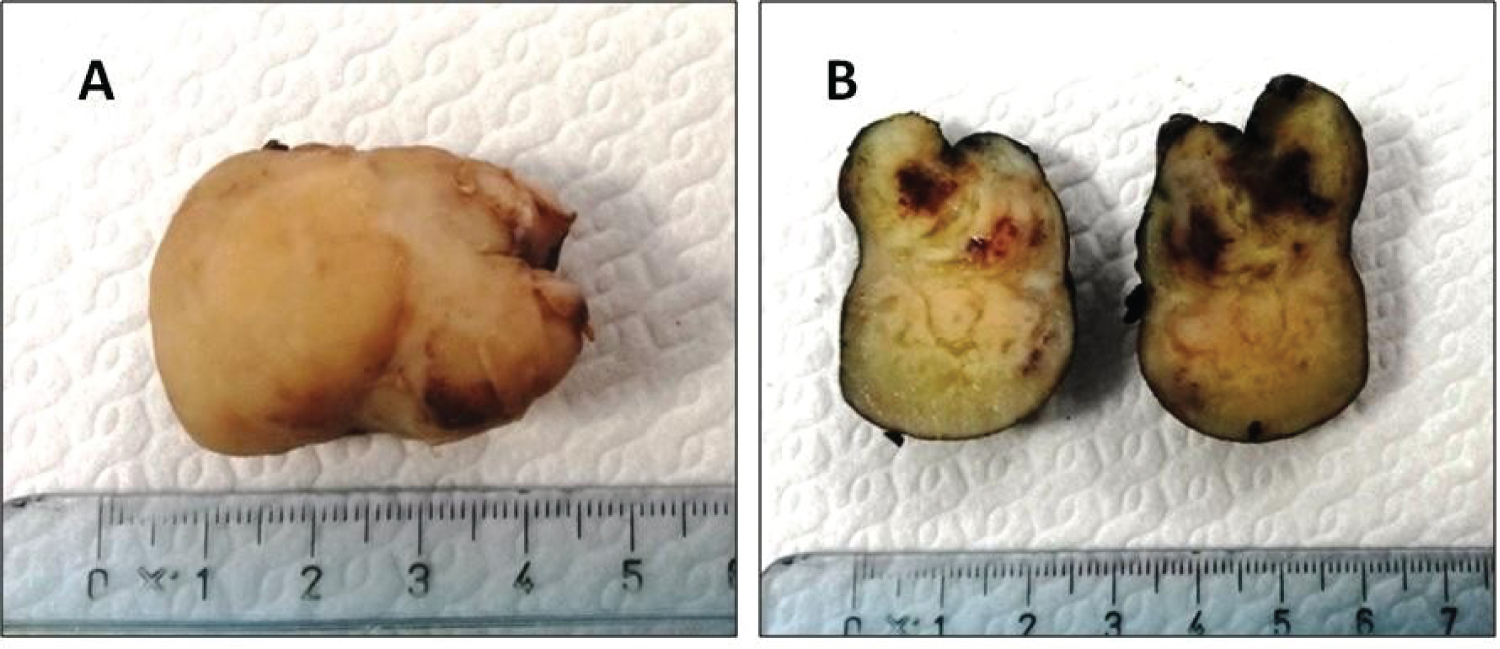 Figure 1: Laryngeal SFT grossly presenting as a firm mass with sharply defined borders (A). The cut surface appeared as heterogeneous and translucent (B).
View Figure 1
Figure 1: Laryngeal SFT grossly presenting as a firm mass with sharply defined borders (A). The cut surface appeared as heterogeneous and translucent (B).
View Figure 1
Collagen bundles were absent. The vascular network was characterized by thin-walled, non-fibrotic vessels with moderately dilated lumina. Tumor necrosis was absent. The proliferation index was calculated with ki67 labeling, resulting 12% in hypercellular areas and 3% in hypocellular areas. Mitotic count resulted in being more than 4/10HPF in the former and less than 4/10HPF in the latter. An immunohistochemical panel, including CD34, bcl-2, STAT-6, smooth muscle actin (SMA), Desmin, citokeratins (AE1/AE3), S100p, PAX8, p53] was performed. Neoplastic cells stained for STAT6, CD34, and bcl-2 (Figure 2). Desmin, SMA, AE1/AE3, PAX8, and S100p were not reactive (not shown). P53 stained at low levels (not shown) (Figure 3). Based on these findings, a diagnosis of SFT, cellular variant, was rendered. Hypercellularity, nuclear pleomorphism, and a raised mitotic count (> 4/10HPF) were considered as atypical features.
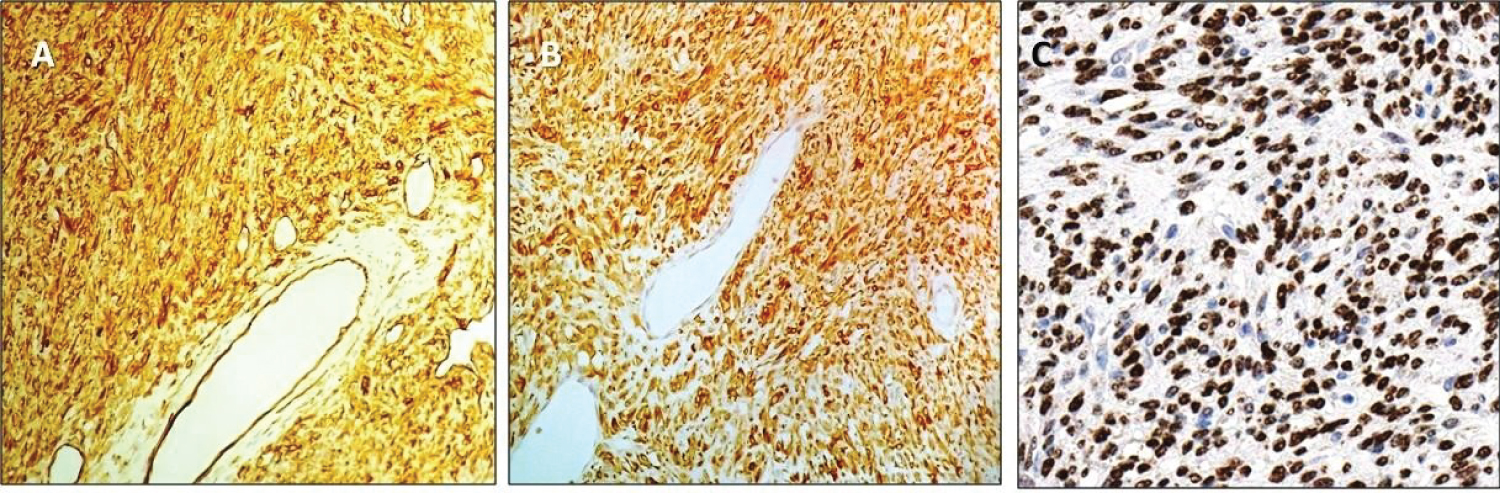 Figure 2: Diffuse immunostaining for CD34; 20x magnification (A) and bcl-2; 20x magnification (B). Nuclear immunolabeling; 40x magnification (C).
View Figure 2
Figure 2: Diffuse immunostaining for CD34; 20x magnification (A) and bcl-2; 20x magnification (B). Nuclear immunolabeling; 40x magnification (C).
View Figure 2
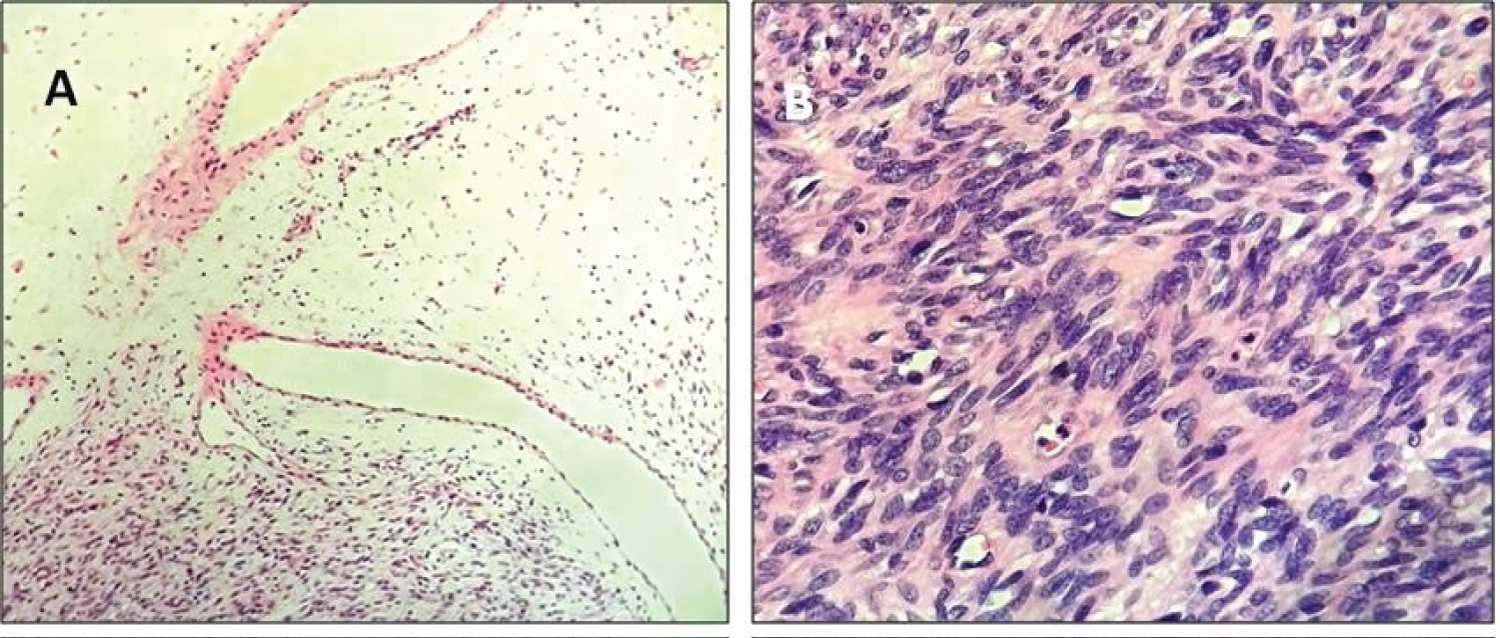 Figure 3: The histological assessment of the SFT's cellular variant showed alternating hypercellular and hypocellular areas of spindle cells proliferation with non-fibrotic dilated vessels; HE, 10x magnification (A); Crowded spindle shaped nuclei with vesicular chromatin; HE, 40x magnification (B).
View Figure 3
Figure 3: The histological assessment of the SFT's cellular variant showed alternating hypercellular and hypocellular areas of spindle cells proliferation with non-fibrotic dilated vessels; HE, 10x magnification (A); Crowded spindle shaped nuclei with vesicular chromatin; HE, 40x magnification (B).
View Figure 3
Molecular analysis revealed the presence of the NAB2-STAT6 fusion gene. Molecular analysis was performed on formalin-fixed paraffin-embedded tissue using the "Archer® FusionPlex Sarcoma panel" on a Personal Genome Machine with Ion Torrent technology (Thermo Fisher Scientific, Life Technologies). This panel detects and identifies fusions of 26 genes associated with soft tissue cancers: ALK, CAMTA1, CCNB3, CIC, EPC1, EWSR1, FOXO1, FUS, GLI1, HMGA2, JAZF1, MEAF6, MKL2, NCOA2, NTRK3, PDGFB, PLAG1, ROS1, SS18, STAT6, TAF15, TCF12, TFE3, TFG, USP6, YWHAE. The tumor was found to harbour the NAB2-STAT6 fusion gene.
Both the clinical management and the diagnostic workflow were approved by our Institutional Head and Neck pathology Group.
The aim of the review is to concisely summarize the clinical-pathological behavior of SFT, a rare cause of laryngeal obstruction, systematically evaluating the clinical-pathological features from previously published case reports. Data from a Medline ranging from 1956 to 2021(NCBI Pubmed) were collected and compared, including a new case report. Overall, 31 cases were collected. Laryngeal squamous cell carcinoma (SCC) and other mesenchymal tumors were excluded.
Since a recent re-classification has been introduced [2,3] and molecular landmarks were shared, cases of both laryngeal SFT and HPC were included in the review, being part of the same spectrum of disease.
SFT and HPC are thought to belong to the same morphological continuum, including SFT's fibrotic (classic) and cellular variant (namely comprising lesions previously ranked as HPC) [3]. Therefore, tumors originally diagnosed as both laryngeal SFT (61%) and HPC (39%) have been considered in the present review [8,9,14,41] (Table 1). Laryngeal HPC shares with SFT the prevalent supraglottic location, clinical presentation, small size, and treatment strategies [30-41].
Table 1: Laryngeal SFT - revised series. According to the current re-classification, 31 lesions previously classified as both SFT and HPC we re included.View Table 1
Overall, both males (64%) and females (36%) are affected, with age ranging from 6 to 79 years, with slight male predominance (about 2:1). Correlation with smoking is inconsistent. The supraglottic region (70%) represents the main site of involvement, with the glottic (23%) and subglottic (7%) locations less frequently affected. In the supraglottic region, the aryepiglottic folds are the prevalent site of involvement (45%), with possible extension to either the piriform sinus or paraglottic space.
In contrast with both pleural and extra-pleural SFT, laryngeal tumors are more likely to be symptomatic. Of note, they have a slow growth rate, gradually compressing the surrounding structures. The presence of local invasion or cartilage and bone erosion should raise the suspicion of malignancy. Symptoms are usually long-standing and can deteriorate toward airways obstruction, a dramatic event that should be urgently managed. At fiber-optic laryngoscopy SFT presentation is variable, ranging from a submucosal ill-defined swelling to a bulky peduncolated mass [20]. Regardless of both the site of involvement and the size of the mass, hoarseness has been frequently found at onset. Additionally, cough, dysphonia, difficulty breathing, foreign body sensation in the throat have been reported [8,9,14,41]. Interestingly, the quality of voice’s impairment was unrelated to altered vocal cord motility. In the subglottic region SFT caused airways' narrowing and dyspnoea [15,18]. Some supraglottic lesions can secondarily involve the hypopharynx, additionally causing dysphagia. In the rare instance in which the hypopharynx is the primary site of involvement, dysphagia dominates the clinical picture, though obstructive respiratory symptoms could also be observed [42,43].
Radiographic imaging is not specific. The tumor shows isoattenuation (relative to the muscle) with enhancement after intravenous contrast injection, on CT imaging. Low attenuation intra-lesional areas were reported to be associated with myxoid or cystic degeneration [41]. On MRI imaging, SFT is iso-intense (relative to the muscle) on T1-weighted images, whereas T2-weighted images can show both homogeneous and heterogeneous enhancement, depending on the different number of fibroblasts and collagen in the tumor composition [44,45]. Interestingly, a correlation has been shown between the hypo-intense signal in T2-weighted images and the hypocellular and collagenous areas [44,45]. Moreover, MRI helps plan surgical management, being the most sensitive procedure in excluding adjacent structures’ invasion, and in evaluating tumor's resectability [24]. Angiography has a low diagnostic reproducibility, being SFT described as hyper- [19] and hypo-vascular [16]. Surgical excision is the treatment of choice. Neither radiation nor chemotherapy plays a role in the management unless malignancy has been established [46]. In the revised series, complete tumor excision was curative in all cases, without evidence of disease during the follow-up. Similarly, those cases defined as atypical due to hyperellularity and increased mitotic count had a disease-free follow-up [18,26].
Furthermore, it should be considered that defining the primary site as laryngeal or hypopharyngeal can affect the surgical approach. Specifically, the surgical approach unfolds as a partial laryngectomy or open lateral thyrotomy in the former, and a transoral excision or lateral pharyngotomy in the latter [20,43].
SFT grossly appears as a solid and firm mass with sharp borders, ranging from a few millimeters to 5-6 centimeters in greatest dimension. The diagnosis rests on the association of both histological and immunohistochemical features. Recently, the concept of HPC as a vascular, pericyte-derived tumor has been abandoned in favor of a fibroblastic origin, thus placing HPC closer to SFT. Lately, these tumors have been reclassified as SFT, in the form of the fibrous variant, containing collagen bundles, fibrous areas, and hyalinized thick-walled vessels, and the cellular variant, characterized by the lack of fibrotic areas, hypercellularity, and thin-walled (non-fibrotic) branching vessels. In many instances, the latter is virtually indistinguishable from HPC [2,3]. Furthermore, several authors have identified a high degree of concordance between the original diagnoses and contemporary reclassification [47]. Immunohistochemistry is useful in confirming the diagnosis. Specifically, the combination of positive bcl-2 and CD34 have been shown to be consistent with SFT [48]. Moreover, CD 99, epithelial membrane antigen (EMA), and smooth muscle actin (SMA) are described as positive in some instances, whereas cytokeratins, S100 protein, and desmin are not reactive. In the past few years, STAT-6 has been found to be associated with SFT. Specifically, a study has identified a recurrent intra-chromosomal paracentric inversion involving the long arm of chromosome 12, resulting in NAB2-STAT6 gene fusion [11]. The resulting chimeric protein was recently identified in SFT, and STAT-6 nuclear immunostain was diffusely positive in such cases [49]. Here we report for the first time the case of a laryngeal SFT where molecular analysis has revealed the presence of the NAB2-STAT6 fusion gene. Accordingly, immunohistochemistry showed diffuse nuclear staining for STAT-6 in almost all tumor cells. TP53 gene mutation has been previously described in SFT, with a diffusely positive immunohistochemical staining in malignant tumors [50]. Conversely, our p53 labeling index was wild type. Moreover, an earlier study has investigated PAX8 expression in SFT. PAX8 is a member of the paired-box family of genes, which resulted positive in a small subset of extra-pleural SFT, especially in the retroperitoneum, while its reactivity was observed in pleural SFT [51]. Accordingly, our case resulted not reactive for PAX8.
SFT should be differentiated by other larynx affecting mesenchymal tumors (e.g., cartilaginous, neurogenic, lipomatous, myogenic and osteogenic tumors). In pediatric patients, both haemangioma and glandular hamartoma can be found. Specifically, the former is more frequently subglottic, while the latter supraglottic [20,52,54]. If malignant SFT is suspected, spindle cell squamous cell carcinoma (SC-SCC) should be excluded. Of note, SC-SCC shares SFT's clinical picture, presenting as a polypoid mass. However, SC-SCC predominantly affects smoker patients.
Importantly, although 30% of SC-SCC could be non-reactive for epithelial markers and, on the contrary, CD34 could be non-reactive in most malignant SFT, SC-SCC shows, at least focally, evidence of squamous differentiation, that is usually absent in SFT [55]. In such instances, differential diagnosis could rely on STAT-6 immunostain, recently identified as SFT's most specific marker [10].
The clinical outcome of both pleural and extra-pleural SFT is unpredictable. Malignant behavior with recurrent disease and distant metastases to lung, liver and bones account of the 10-15% for extrapleural tumors. The metastatic rate is higher for the cellular rather than the fibrotic variant [46,56]. Atypical forms of SFT have been described in both thoracic and extra-thoracic locations [2]. Atypia is qualified abnormal by nuclear features, including coarse chromatin, anisonucleosis, more than mild pleomorphism and nuclear overlap [47]. Additionally, other atypical features include increased mitotic activity (greater than 4 mitoses/10 HPF) and tumor necrosis [57]. Hypercellularity has been mentioned among the atypical features. However, some authors have considered its assessment as arbitrary [12].
Furthermore, the presence of anaplastic/dedifferentiated areas have been highlighted as an additional criterion in establishing the malignant potential in both pleural and extra-pleural SFT [58-62]. The proposed diagnostic criteria for malignancy are summarized in Table 2. Interpretation of such criteria in biopsy specimens should be performed, made with caution to avoid an underestimation of the malignancy risk with a partial tumor evaluation. Therefore, evaluating the whole surgical specimen is advisable. Both immunohistochemical markers and molecular tests failed as prognostic predictors, and a weak connection between morphology and clinical outcome has been observed in both extra-pleural SFT's variants [58,62,64]. Conversely, malignant behavior has not been reported in the larynx. Atypical histological features (e.g. hypercellularity and raised mitotic activity) are exceedingly rare in the larynx [18,26]. The present is the third case of atypical laryngeal SFT, although the term ‘‘atypical’’ does not determine the tumor's behavior. Hence, atypical histological features were found in about 10% of all the revised cases and were not correlated with local recurrences or malignant behavior.
Table 2: Summary of SFT's clinical and histologic diagnostic criteria.View Table 2
According to the most widely used risk stratification model, older age, tumor size, increased mitotic activity and tumor necrosis correlate with clinical outcome in SFT. Of note, tumor size has a great prognostic value, correlating with the presence of a malignant component and affecting tumor's respectability [12]. In contrast with other extra-pleural sites, the larynx does not harbor lesions greater than 5-6 centimeters. Such finding may be explained by the larynx's anatomy, that could be responsible for the early symptomatic onset and the benign clinical behavior and the very low rate of local recurrences. Additionally, tumor’s respectability could be affected by infiltrative margins. Specifically, these may trap native structures and prevent a complete surgical excision [47,63,64]. A correlation has been observed between incomplete tumor resection and local recurrences' rate (one of SFT's most reliable predictors of clinical outcome) [65]. An increased mitotic activity has been linked to high risk of metastases and death of disease [46,65]. While a mitotic count greater than 4/10 HPF is crucial in discriminating both atypical tumors and aggressive behavior, it cannot be used as a unique marker. As described here, an increased mitotic activity represents an atypical feature. However, the revised data showed it is insufficient in driving the clinical behavior toward malignancy. Additionally, both tumor necrosis and cartilage or, more rarely, bone infiltration also suggest SFT's malignant potential [12,50,58]. Finally, SFT's primary site has been reported to predict the outcome. Specifically, pleural tumors tend to develop local (intra-pleural and mediastinal) metastases, with a low rate of distant metastases. In contrast, abdominal tumors behave more aggressively, with both extra-peritoneal and multiorgan metastases [64-67]. Conversely, the laryngeal location was characterized by a benign clinical behavior.
Both fibrotic and cellular variants of SFT have been reported in the larynx. Laryngeal SFT represents a rare cause of airway obstruction, reported only in 31 cases. It is characterized by non-specific clinical and radiologic features therefore, when SFT is clinically suspected, a histological assessment is advisable. The NAB2-STAT6 gene fusion and nuclear immunostaining for STAT-6 are useful in confirming the diagnosis. Importantly, the present review highlighted that laryngeal SFT displays a very peculiar clinical behavior vs. other involvement sites, resulting in five features: 1) Early symptomatic onset 2) Small size at diagnosis 3) Prevalent supra-glottic location 4) Impairment of the quality of voice with vocal muscle sparing 5) Favorable clinical outcome. The tumor usually behaves benignly, since atypical histological features were uncommon (10% of cases) and unrelated to local recurrence or metastasis (Table 3). The finding of increased mitotic activity alone was unsuitable in predicting the SFT aggressiveness in the larynx. Thus, the proposed risk stratification models resulted not suitable for the laryngeal SFT. Nonetheless, the clinical behavior remained unpredictable. Hence a long-term follow-up is advisable for all patients.
Table 3: The table summarizes both the clinical and pathological features in 31 cases of laryngeal SFT.View Table 3
The authors received no financial support for the research, authorship and publication of this article.
The authors declared no potential conflict of interest.
Authors did not receive any financial support.