Background: Nasal valve collapse is a primary cause of nasal obstruction. Patients with nasal airway obstruction suffer from a variety of symptoms that affect quality of life including congestion, headache, sleep problems, daytime sleepiness, and snoring.
Objective: To evaluate the effectiveness of a low-power temperature-controlled radiofrequency procedure to treat the nasal valve and measure symptomatic improvement in patients diagnosed with nasal airway obstruction due to nasal valve collapse.
Methods: A prospective, single-arm, multi-institutional study at 12 otolaryngology centers across the United States in adults > 18 years suffering from nasal airway obstruction that responded to temporary nasal valve dilation, and with a baseline Nasal Obstruction Symptom Evaluation (NOSE) scale score ≥ 60. Patients were treated in the nasal valve region with temperature-controlled radiofrequency energy and followed up at 3 months.
Results: 122 adult patients underwent the procedure and 3 patients were lost to follow-up at the 3 months visit. NOSE scale total scores at three months post-procedure were significantly improved relative to baseline, from 80.3 (± 12.6; range: 60-100) to 32.9 (± 24.2; range: 0-100), P < 0.001. At baseline, 100% of patients' total NOSE scale scores were in the 'extreme' (score of 80-100) or 'severe' (55-75) categories; at three months post-procedure this decreased to 18.5%. At the three-month visit, 91.6% of the patients had either a 20% improvement in NOSE scale total score relative to baseline or at least one severity category improvement.
Conclusion: Minimally-invasive temperature-controlled radiofrequency treatment of the internal nasal valve led to significant improvement in NOSE scale scores at three months. This treatment is safe and efficacious to treat nasal airway obstruction due to valve collapse.
Nasal valve, Nasal obstruction, Radiofrequency, Nasal congestion, NOSE scale
NOSE: Nasal Obstruction Symptom Evaluation; RF: Radiofrequency; VAS: Visual Analog Scale; SD: Standard Deviation; ANCOVA: Analysis of Covariance
Chronic nasal obstruction is a common health condition often associated with nasal congestion, stuffiness, headache, fatigue, sleep disturbance, daytime sleepiness, and snoring. These issues may lead to a decreased quality of life [1,2]. The internal nasal valve area - bounded by the dorsal septum, the caudal end of the upper lateral cartilage, and the head of the inferior turbinate - creates the greatest airflow resistance in the nasal airway [3]. Common, independent anatomical contributors to obstruction include nasal valve collapse, septal deviation, and turbinate hypertrophy, and even slight constriction results in an exponential increase in resistance. Among nasal obstruction patients with severe or extreme symptoms, 73% have nasal valve collapse as a contributor [4].
Mechanical dilators (such as over-the-counter external nasal strips and in-nostril stents or clips) are common first-line treatments for nasal valve collapse. Otolaryngologists may consider surgery for intractable cases [5-7], with aims to open and/or strengthen the lateral nasal wall [8]. The main surgical approaches include cartilage grafting, suspension techniques, and patency-maintaining implants. These techniques require incisions and are often technically complex, and carry risks of bleeding, implant/graft extrusion, infection, cosmetic changes, and persistent discomfort, along with costs of procedures performed in higher-acuity operating room settings [9].
There is a need for straightforward, incisionless, minimally invasive procedures to correct nasal valve collapse, which may confer less clinical risk and enable procedures to be performed in a lower-acuity office setting. Temperature-controlled radiofrequency (RF) treatment of the nasal valve has previously been reported as means to achieve symptomatic improvement [10-12]. This study's objective was to evaluate the effectiveness of temperature-controlled RF treatment of the nasal valve in an observational cohort of patients diagnosed with nasal airway obstruction due to nasal valve collapse.
This was a prospective, single-arm, open-label, multi-center study conducted at 12 locations across the United States and registered on clinicaltrials.gov (NCT04277507). All study activities had the approval and oversight of the Western Institutional Review Board (study ID: 20192967). All site investigators were board-certified otolaryngologists and participants were recruited from their clinical practices. Patients gave their written informed consent prior to any study activities.
Patients 18 years of age and older were included on the basis of valve-related nasal obstruction, score on the validated Nasal Obstruction Symptom Evaluation (NOSE) scale of at least 60 (where 100 means the worst possible reported symptoms of nasal obstruction [13]), and positive response to modified Cottle maneuver or other temporary nasal valve dilation or stabilizing measures. Participants were excluded by prior nasal valve surgery within 6 months or medical contraindications. Full eligibility criteria are presented in Table 1.
Table 1: Inclusion and exclusion criteria. View Table 1
At baseline, data collection and assessments included demographics, medical history, NOSE scale, examination of the nasal area, and endoscopicnasal examination including photographic/video imaging. Patients continued using their usual medications such as topical nasal steroids throughout the study.
The device, treatment, and potential mechanism of action of the RF treatment have been previously described [10,11]. The Aerin console and single-use Vivaer Stylus comprise the Vivaer System. The console delivers temperature-controlled RF energy to the stylus, which conducts bipolar RF energy to target tissue through eight electrodes. The device controls energy delivery by monitoring tissue temperature and automatically adjusting the RF current to maintain a therapeutic treatment temperature of approximately 60 °C.
Topical and local anesthesia were administered prior to RF treatment with the agent, dose, and volume per investigator preference and patient needs. The stylus was placed on the lateral wall of the nasal valve and treatment was applied to the mucosal tissue near the caudal end of the upper lateral cartilage at non-overlapping loci. Treatment proceeded with default treatment settings: Temperature, 60 °C; power, 4 watts; treatment time, 18 seconds treatment time (could be varied from 10-20 seconds based on case-specific needs); cooling time, 12 seconds. Treatment sites were evaluated post-procedure via endoscopy.
At three months post-procedure, patients repeated the NOSE scale, examination of the nasal area, and endoscopic nasal examination including photographic/video imaging. Patients also answered yes/no satisfaction questions and marked a standard 100-mm visual analog scale (VAS) to characterize their pain intensity at the treatment area. Adverse events were recorded from the day of procedure through three months.
Data were evaluated in post hoc analysis. Missing data were not imputed. Unless otherwise noted, data are presented as means ± standard deviation (SD), ranges, and percent of total. NOSE scale data are presented as descriptive statistics of the total score and component scores, as well as severity classifications [14]. Responders were defined as those patients with either 20% improvement in total NOSE scale score from baseline or at least one severity category improvement [15]. Statistical testing used paired t-tests or analysis of covariance (ANCOVA) with significance set at p < 0.05 with no adjustment for multiple comparisons.
Between February 2020 and August 2020, 122 patients were treated with the RF system. The group included slightly more women than men, with an average age of 50 years (range, 19-83 years). All patients suffered from nasal obstruction due to dynamic and/or static nasal valve collapse. Nasal obstruction had been chronic for at least a year in 96% of patients. Nearly half of the patents (n = 57) had prior nasal obstruction procedures. Baseline demographics and baseline characteristics are shown in Table 2.
Table 2: Patient demographics and baseline characteristics. View Table 2
Table 3 outlines the procedure specifics. Lidocaine with epinephrine was the most-commonly used anesthetic. All patients were treated bilaterally, with the exception of one unilateral case. Treatment was applied at 1-10 sites per side, with 91.3% of patients having three, four, or five treatment sites per side. The default device settings were used in 96% of cases; the exceptions applied treatment times for either 17 or 20 seconds, instead of the default 18 seconds.
Table 3: Procedural data. View Table 3
At three months post-procedure, 119 patients were evaluated (two patients were unavailable due to COVID-19 pandemic-related travel restrictions and one was lost to follow-up). Mean NOSE scale total scores at three months post-procedure were significantly improved relative to baseline, from 80.3 (± 12.6; range: 60-100) to 32.9 (± 24.2; range: 0-100) (P < 0.001; Figure 1). This was a 59.0% improvement. At baseline, 100% of patients had total NOSE scale scores classified as 'extreme' (score of 80-100) or 'severe' (55-75) [14]; at three months post-procedure this decreased to 18.4% (Figure 2).
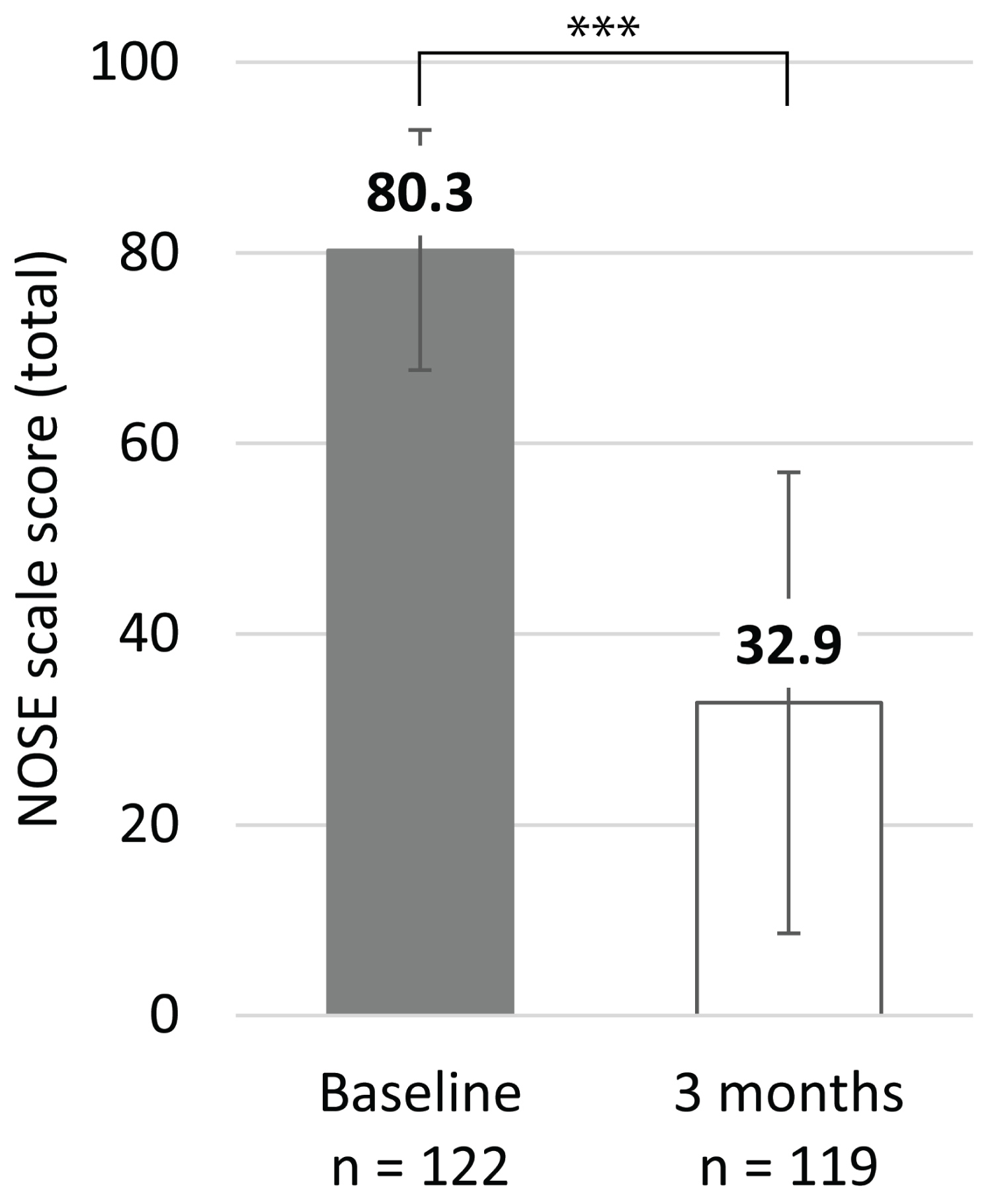 Figure 1: Mean NOSE scale total scores at baseline and three months.
Figure 1: Mean NOSE scale total scores at baseline and three months.
Bars represent the SD; *** indicates P < 0.001.
View Figure 1
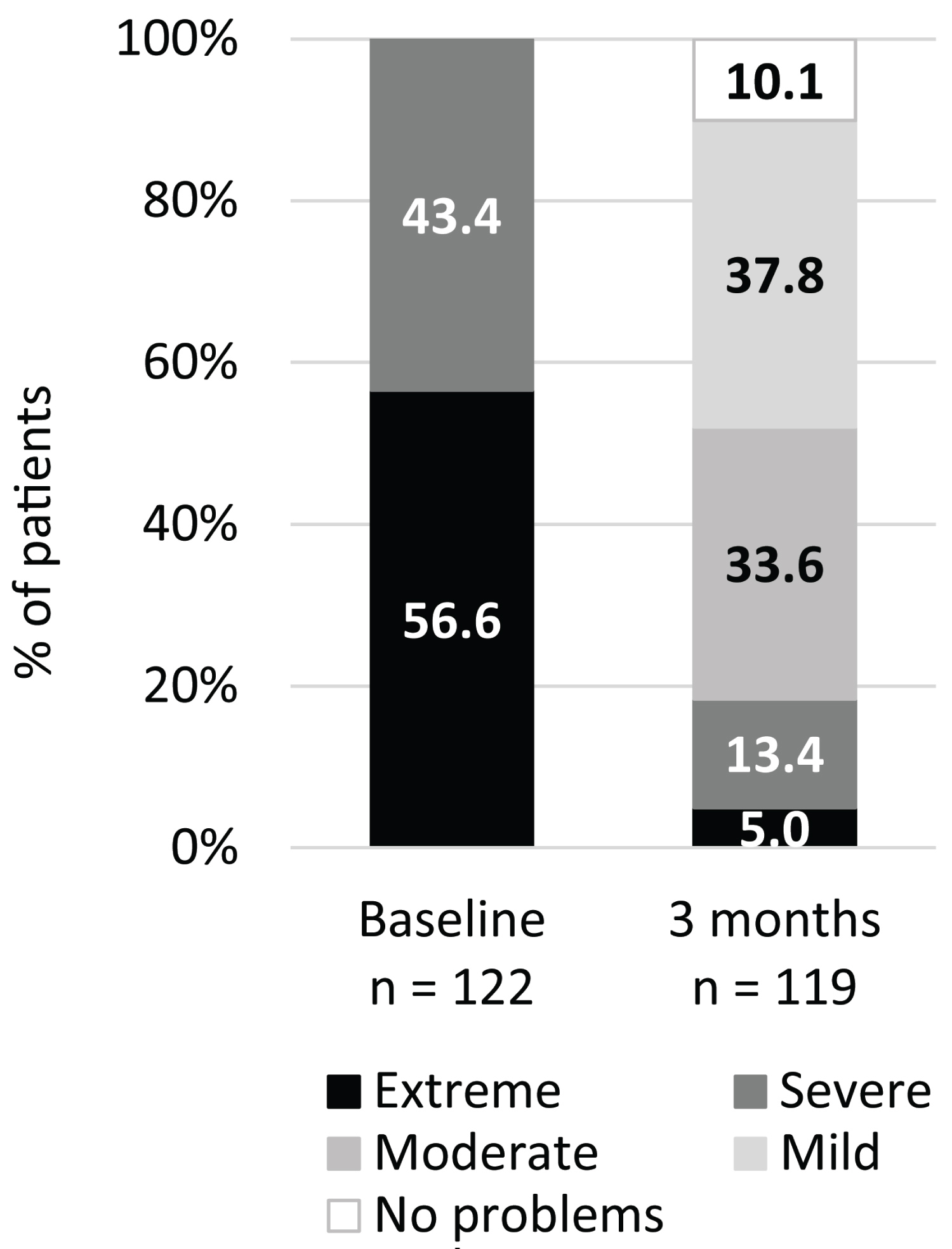 Figure 2: Proportion of patients in each of the NOSE scale severity classification system, based on total score, at baseline and three months. Extreme (80-100), Severe (55-75), Moderate (30-50), Mild (5-25), No problems (0-5).
View Figure 2
Figure 2: Proportion of patients in each of the NOSE scale severity classification system, based on total score, at baseline and three months. Extreme (80-100), Severe (55-75), Moderate (30-50), Mild (5-25), No problems (0-5).
View Figure 2
Each NOSE scale component score also improved significantly at the three-month follow-up (P < 0.001 relative to baseline for all component scores; Figure 3). The distribution of scores for each of the NOSE scale components also illustrates the consistent improvement (Figure 4).
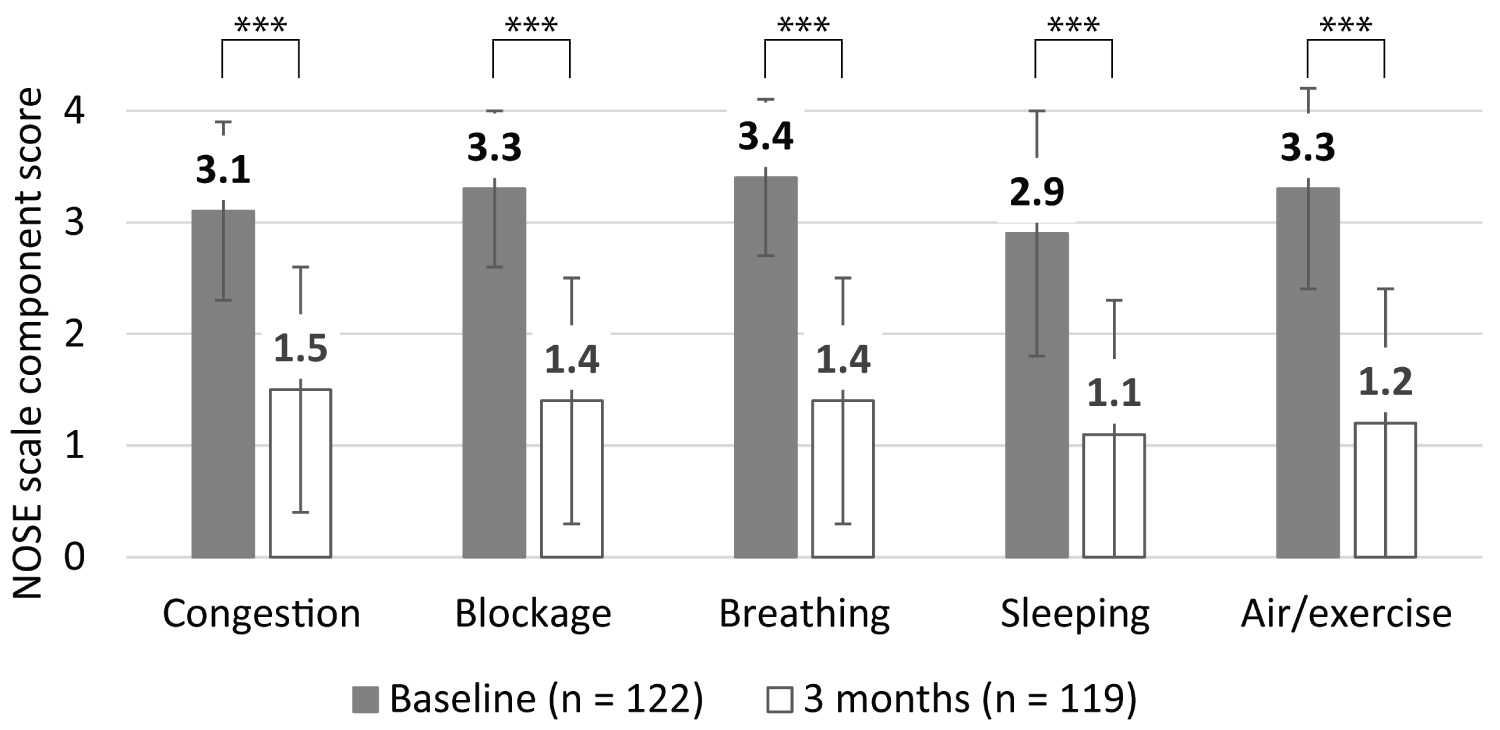 Figure 3: Mean NOSE scale component scores at baseline and three months.
Figure 3: Mean NOSE scale component scores at baseline and three months.
Bars represent the SD; *** indicates P < 0.001.
View Figure 3
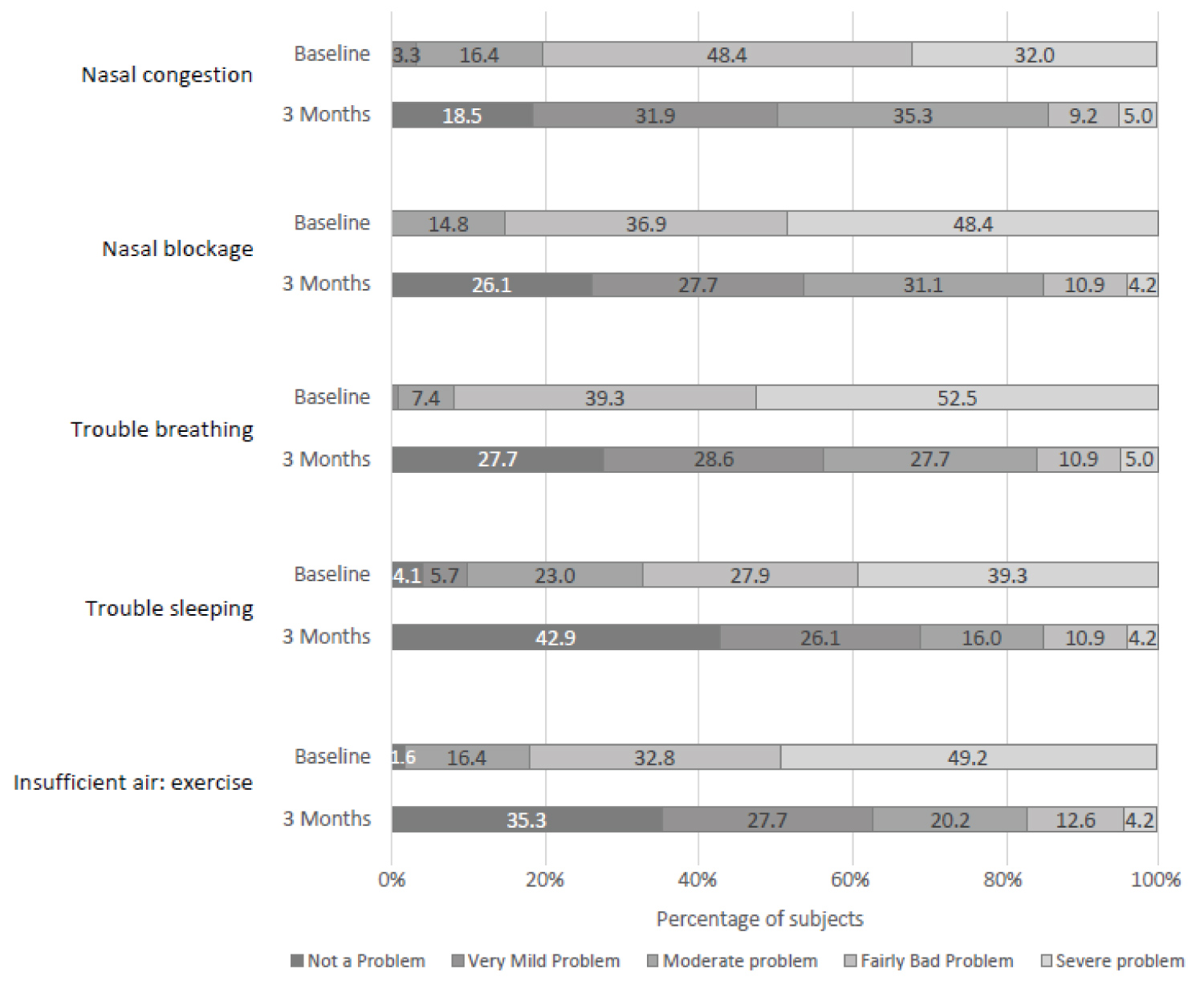 Figure 4: Proportion of patients with the different NOSE componentscores at baseline and three months. Severe problem (4), Fairly bad problem (3), Moderate problem (2), Very mild problem (1), No problem (0).
View Figure 4
Figure 4: Proportion of patients with the different NOSE componentscores at baseline and three months. Severe problem (4), Fairly bad problem (3), Moderate problem (2), Very mild problem (1), No problem (0).
View Figure 4
NOSE scale score improvements were statistically significant independent of patients' history of previous nasal surgery. For patients with prior nasal surgery and both baseline and three-month scores (n = 65), mean total NOSE scale scores were 82.6 (± 12.3; range: 60-100) at baseline and 36.3 (± 24.7; range: 0-100) at three months, while for patients without a history of nasal surgery and both baseline and three-month scores (n = 54), mean total NOSE scale scores were 78.0 (± 12.6; range: 60-100) at baseline and 28.8 (± 23.3; range: 0-95) at three months. ANCOVA on change from baseline using baseline as the covariate demonstrated no significant difference between the groups.
At three months, the proportion of patients with either 20% improvement in NOSE scale total score relative to baseline or at least one severity category improvement was 91.6%, with a 95% confidence interval of 85.2% to 95.4% (Figure 5 shows the distribution of percent change in NOSE scale score from baseline for the population and differentiates the responders and non-responders, as defined). Of the 10 patients who were non-responders, five had a history of nasal surgery, two had enlarged turbinates, and two had bilateral septal deviations that were not addressed.
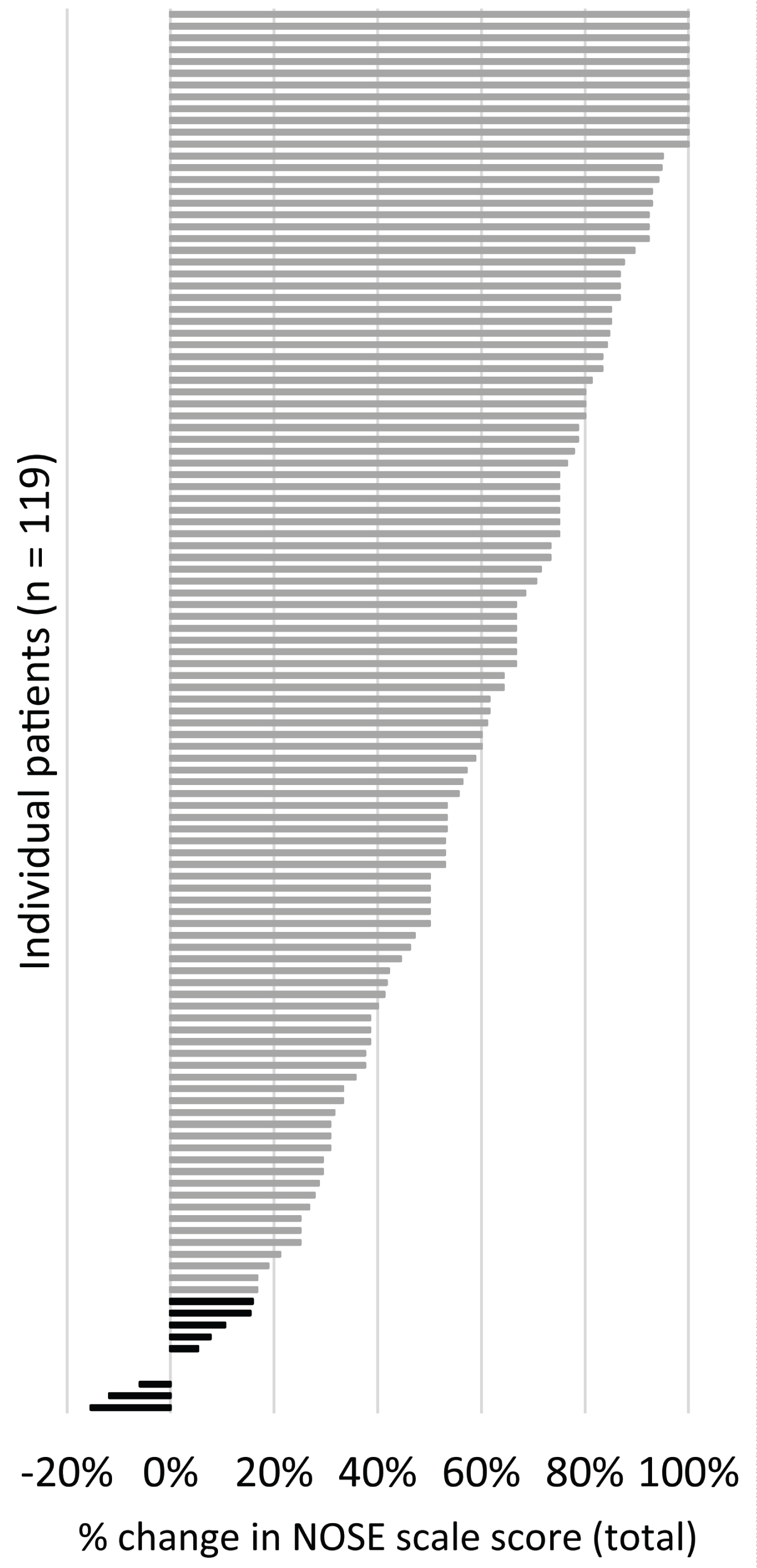 Figure 5: Tornado plot showing the percent change in total NOSE scale score between baseline and three months for each of the patients, where a positive percent change is animprovement (decrease) in NOSE scale score. Non-responders are in black, responders are in grey.
View Figure 5
Figure 5: Tornado plot showing the percent change in total NOSE scale score between baseline and three months for each of the patients, where a positive percent change is animprovement (decrease) in NOSE scale score. Non-responders are in black, responders are in grey.
View Figure 5
Given yes/no choices regarding satisfaction, mostpatients (87.3% of n = 118 with data) indicated that they would recommend the procedure to a friend, and 73.7% would repeat the procedure if their symptoms returned.
Mean treatment site pain intensity at three months post-procedure was 4.5 (± 8.8; range: 0-60) out of a maximum of 100. No patients required narcotic pain relief following the procedure. Physical and endoscopic exam results did not reveal concerning side effects or damage to neighboring tissue. During the study, there were no serious adverse events related to the device or study procedure. Ten adverse events that were considered related to the device or study procedure occurred. There were eight reports of nasal/sinus tenderness, crusting, and/or pressure/congestion; seven were moderate, one was severe, and all resolved during the study period. One patient experienced a moderate sinus infection after the procedure, which resolved with antibiotic treatment. One patient had a mild transient vasovagal syncope response to the injectable anesthesia. Additionally, a single severe adverse event occurred but was considered unrelated to the device or study procedure: A patient was hospitalized due to a urinary tract infection.
Endoscopic assessment immediately following the procedure revealed that 5.0% of treatment sites had mild bleeding on both sides, which resolved without further intervention. At three months post-procedure, no patients had undergone additional treatments with the RF stylus nor had any nasal surgical interventions since baseline.
Nasal valve surgery has historically been reserved for patients with the most severe nasal valve collapse due to the complexity, cost and morbidity of surgical intervention. Given the improvement in chronic nasal obstruction shown by nasal valve surgery, a less invasive, in-office procedure targeting the nasal valve has the potential to allow broader access to needed nasal valve treatment.
Lateral wall procedures can be challenging, due to risks of collapse of the alar rim or lateral crura, graft resorption or migration, scarring, and graft necrosis [16,17]. In meta-analyses of functional rhinoplasty, NOSE scale score reductions of 49.8 points [18] and 47.7 points [19] have been reported. As a minimally invasive procedure, RF treatment of nasal valve collapse is likely to avoid these complications, while delivering comparable efficacy outcomes based on the reported reductions in NOSE scale scores.
This study of 122 patients demonstrates that in-office treatment of nasal valve collapse with temperature-controlled RF was effective because a statistically significant improvement in the symptoms of nasal obstruction were observed. At three months after treatment, 91.6% of patients had a positive response to temperature-controlled RF treatment of nasal valve collapse. Total NOSE scale scores decreased by 59.0%. Treatment was effective in patients who had previous surgeries. Previous reports using treatment with the same device yielded similar results at three months after treatment: Total NOSE scale score reduction of 54% in 31 patients [12] and 66% in 50 patients [10]. In this report, the procedure was generally well-tolerated, and patients were satisfied with their outcomes. The cohort in the current study was much larger than previous studies and contributes to converging trends across multiple trials, which show that patients experience significant relief in symptoms by three months.
This study used the NOSE scale as its key clinical measure. The NOSE scale is a validated and highly sensitive scale that is the gold standard for assessing nasal obstruction symptom burden [13,14]. Its minimum clinically important difference is 30 points [20]; therefore, the 47.4-point observed improvement in this study exceeds that benchmark. A treatment responder was defined as either a 20% improvement or at least one severity category improvement. At three months post-procedure, 91.6% of patients met those criteria.
As evident in the data, a minority of patients did not respond to treatment. There are several possibilities for the lack of improvement which include the presence of concurrent obstructive pathologies such as a septal deviation and turbinate hypertrophy. These areas of obstruction can contribute to a significant source of nasal obstruction as they were not addressed with the temperature-controlled RF procedure of the nasal valve. Since the overall responder rate was 91.6%, it is important to note that many patients even with concurrent obstructive pathologies noticed a significant improvement in symptoms without intervention of other areas.
Relative to surgical approaches, the temperature-controlled RF procedure is minimally invasive, simple to perform, and low risk to patients. Furthermore, an independent study on cadaver heads concluded that the procedure does not add additional aerosol particulates, which is an important consideration for providers in the era of the COVID-19 pandemic [21]. Minimally invasive techniques enabling procedures in lower-acuity office settings provide new options that may be appealing to clinicians and patients and present the potential for more efficient care benefitting our healthcare system. Based on the high rates of satisfaction among patients, it can be surmised that resolution of their nasal obstruction symptoms may be associated with concomitant improvements in quality of life domains such as quality of sleep, breathing during exercise, embarrassment, snoring, and mouth breathing, as have been described in previous clinical trials for nasal obstruction [1,2,22,23].
None of the patients in this study had additional nasal surgery through three months follow-up. However, additional procedures, if needed, would not be precluded or need to be delayed after temperature-controlled RF treatment as it has minimal impact on the soft tissue.
With respect to patient safety, the VAS pain scores were acceptable, with the intensity of mean residual pain at three months rated 4.5 out of 100 and easily categorized as 'mild' [24]. Bleeding at the treatment site was minimal post-procedure and any instances were fully resolved without additional interventions. Physical and endoscopic exam results did not reveal any unexpected external physical or internal intranasal changes. The frequency of device- or procedure-related adverse events was relatively low and mostly involved post-procedural discomfort and swelling that readily resolved, not raising significant concern.
A limitation of this study is its lack of a control arm. Therefore, outcome reporting may be biased and improvement in symptoms scores could be partly due to a placebo effect. Future randomized controlled trials will be needed. Moreover, three-month outcomes reflect the acute effect but remain preliminary. However, previous work has shown that three-month data is predictive of long-term follow-up [10,11] and it is expected that these results will be similarly durable. Patients continue to contribute data in this study and future reports will evaluate the durability of the longer-term treatment effect.
The results of this large single-arm study add to the body of evidence that minimally invasive temperature-controlled RF treatment of the internal nasal valve to address nasal airway obstruction is safe and efficacious. There was significant improvement in NOSE scale scores at three months. Longer-term follow-up is warranted to further evaluate the durability of the effect.
The authors would like to thank study site principal investigators: Jeffrey Aroesty, MD; Daniel Charous, MD; Andrew Courson, MD; Adil Fatakia, MD; Dale Ehmer, MD; Curtis Johnson, DO; Nathan Nachlas, MD; Jordan Pritikin, MD; and Michael Sillers, MD. The authors thank Jeff F. Doerzbacher, MS for assistance in the statistical analysis and Allison Foster, PhD for writing assistance.
WCY serves as a consultant for Stryker (Kalamazoo, MI) and on the Speaker's Bureau for Optinose (Yardley, PA). RAO serves as a consultant to Aerin Medical, Intersect ENT, Lyra Therapeutics, Optinose, and Stryker. RAO also serves on the Speaker's Bureau for Optinose. HPB serves as a consultant for Aerin Medical.
The study was funded by Aerin Medical. The author(s) received no financial support for the authorship and/or publication of this article.