Peripheral facial nerve palsy is mostly attributed to Bell's palsy, however in 5-10% of cases it may be from an underlying malignancy. It can be difficult to distinguish initially, frequently leading to misdiagnosis and a delay in management, potentially compromising survival of these patients. A common distinguishing feature is that facial palsy from malignancy is typically gradual onset and progressive as opposed to sudden in that of Bell's palsy, although other benign causes may also present similarly. Magnetic resonance imaging is the imaging modality of choice, but it can be falsely negative, adding to the diagnostic difficulty. We present four cases encountered in our department to illustrate the challenges in managing these patients who ultimately were all diagnosed with a malignant cause for their facial palsy. Following a review of literature and from our experience, we propose a more proactive approach to expedite high risk patients to a diagnostic nerve exploration to avoid delays in management.
Facial palsy, Parotid neoplasm, Neoplasm unknown origin, Squamous cell carcinoma
A peripheral facial nerve palsy can be due to many causes, with 80% attributed to Bell's palsy which is idiopathic, although autoimmune and viral causes have been implicated. 70% of these cases completely recover. Bell's palsy is characterized by a sudden onset of complete unilateral lower motor neuron facial palsy with early recovery of function. When the onset of a palsy is gradual and the weakness is progressive, this may be an indication of underlying malignancy. In 5-10% of cases of peripheral facial palsy due to malignancy it can be difficult to identify the cause [1,2]. We describe our experience with four cases of occult malignancy presenting as a facial nerve palsy and review the relevant literature.
This is a presentation of a case series encountered by the authors' institution followed by a review of literature. Medline was searched with the terms "facial paralysis" AND "carcinoma", "SCC", "parotid neoplasm", OR "neoplasms, unknown primary" between 1 Jan 2000 to 18 Nov 2020 in the English language. A total of 16 relevant papers were included.
An 81-year-old man with a background of hypertension and no history of previous skin malignancies presented to the neurology department one year after onset of a progressive left lower motor neuron facial weakness. Magnetic resonance imaging (MRI) scan performed a year after onset showed no abnormality of the facial nerve. He received ectropion repair of the lower eye lid, brow lift, and tarsorrhaphy by an ophthalmologist. When seen by an otolaryngologist two years after onset, a repeat MRI with contrast showed oedema and hyperemia along the maxillary (V2) and mandibular (V3) divisions of the trigeminal nerve extending to involve the foramen ovale and the facial nerve up to the mastoid segment (Figure 1). It also showed enhancement of the parotid gland but without any discrete lesion. By now he had complete left facial palsy, numbness of lip, face, tip of tongue, and gums on left side. He subsequently had a non-targeted ultrasound-guided core biopsy of the parotid gland which demonstrated a primary salivary gland cancer, favoring salivary duct carcinoma. Multidisciplinary meeting recommended palliative radiotherapy, which he managed to only partially complete due to side effects, including neuropathic pain, lacrimation, hypersalivation, and fatigue. He also had facial reanimation with a static sling with tensor fascia lata, as well as gold weight to the upper eyelid. A year following diagnosis he was found to have metastasis to C6 vertebrae with associated paresthesia of the right arm and received radiation therapy to the lesion. He remains alive nearly four years following onset of symptoms.
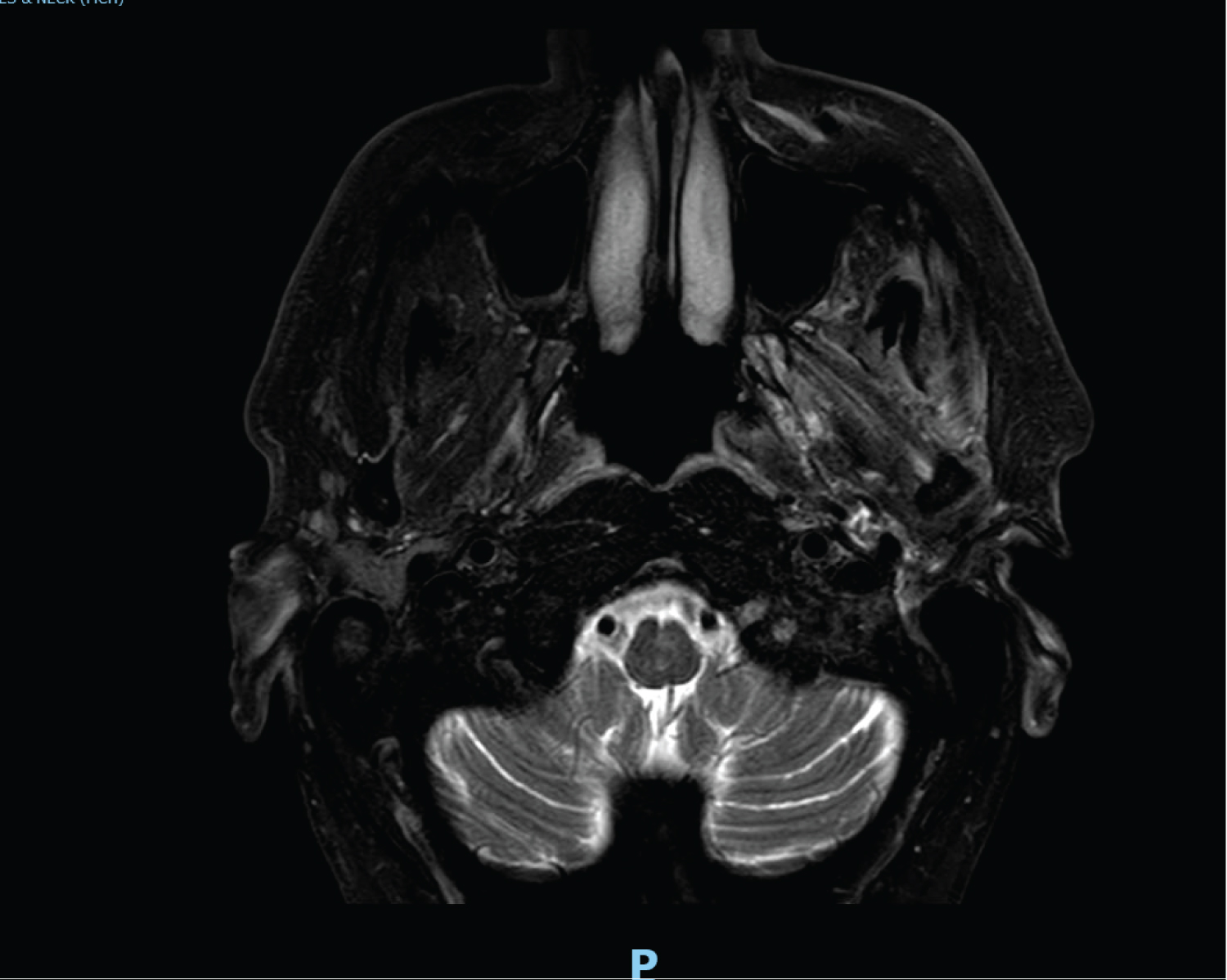 Figure 1: MRI of case 1 showing enhancement of left masticator muscles implicating involvement of trigeminal nerve.
View Figure 1
Figure 1: MRI of case 1 showing enhancement of left masticator muscles implicating involvement of trigeminal nerve.
View Figure 1
A 60-year-old man presented to otolaryngology with a 4-month history of progressive left sided facial weakness and numbness associated with tingling in the left nasolabial fold and reduced sensation along the course of V2 and V3. He is otherwise well, a non-smoker, and does not have a history of any cutaneous lesions. MRI scan at five months after onset was negative, as were his CSF samples and non-targeted core biopsy of his parotid gland. Nerve conduction study showed no activity in frontal and buccal branches with minimal activity of the marginal mandibular branch. A repeat MRI ten months after onset suggested facial nerve enhancement in the stylomastoid foramen and the mastoid segment (Figure 2). He underwent total parotidectomy and intra-operatively the main trunk of the facial nerve was found to be thickened and abnormal. Frozen section confirmed malignancy. The nerve was resected up to the first genu, but the frozen section margin was still positive. He had facial nerve reanimation with tensor fascia lata sling and masseteric nerve transfer to the buccal branch of the facial nerve. Final histology showed squamous cell carcinoma (SCC) with intra and perineural invasion, but no parotid malignancy. He further had resection of the nerve via translabyrinthine approach to the brain stem, with a clear resection margin. He received post-operative radiotherapy of 60 Gy and remains alive with good dynamic movement of the buccal branch of the facial nerve.
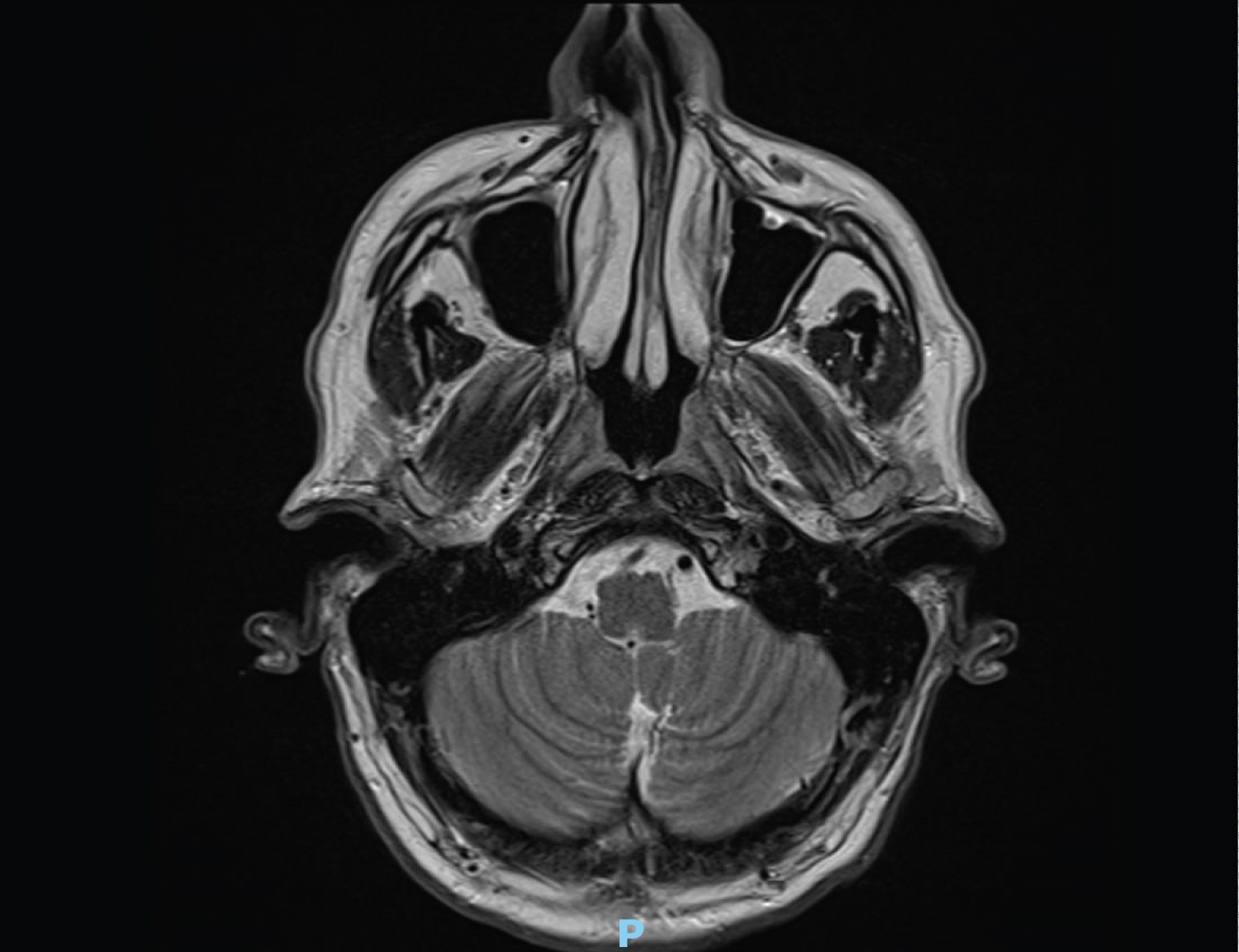 Figure 2: MRI of Case 2 showing abnormal enhancement of left facial nerve.
View Figure 2
Figure 2: MRI of Case 2 showing abnormal enhancement of left facial nerve.
View Figure 2
A 56-year-old man with a background of previous right cheek SCC completely excised 4 years earlier initially presented to otolaryngology department with a right-sided progressive facial nerve palsy. The presentation was unusual in that the palsy started the day after a temporomandibular joint procedure to which it was initially attributed. An MRI one month after presentation, and again three months later, was normal, as was another MRI done soon after a diagnostic facial nerve biopsy, done about five months after the 2nd MRI. He also developed numbness in the trigeminal nerve distribution. The biopsy of the buccal branch of the facial nerve, the first to be completely paralyzed, demonstrated adenosquamous carcinoma. He underwent radical parotidectomy and resection of the facial nerve up to the second genu, followed by curative intent radical radiotherapy. Histology showed a small adenosquamous carcinoma in the parotid gland. Facial reanimation procedures were subsequently performed. Four years after initial specialist presentation he reported headaches and otalgia a further MRI did not reveal any recurrence. The following year he developed dysphagia which progressed to reduced pharyngeal sensation, and diplopia, due to recurrent laryngeal nerve palsy and oculomotor palsy, respectively. MRI confirmed perineural involvement with enhancement in Meckel's cave extending close to the pons (Figure 3). This was the first time an MRI had shown the pathology. He passed away about 6 years following initial presentation.
 Figure 3: MRI of Case 3 showing enhancement of right pons.
View Figure 3
Figure 3: MRI of Case 3 showing enhancement of right pons.
View Figure 3
A 76-year-old man with a background of chronic lymphocytic leukemia (CLL) and no history of previous skin cancer presented with a right sided facial nerve weakness progressing to complete palsy over two months. There was no associated pain, numbness, or involvement of other cranial nerves. His initial MRI did not reveal any cause except neck nodes related to his CLL. Nerve conduction studies showed only minor reinnervation features. Ultrasound-guided core biopsy of the normal parotid gland was normal. Approximately five months after onset he presented with headaches and behavioral changes. Lumbar puncture sample was negative, however MRI revealed enhancement of the facial nerve extending to the internal auditory meatus (Figure 4). He subsequently had a superficial parotidectomy and biopsy of the facial nerve which demonstrated perineural invasion by both spindle cell SCC and CLL. He was treated with palliative intent radiotherapy and remains alive 12 months after the onset of facial weakness.
 Figure 4: MRI of Case 4 showing enhancement of facial nerve in internal auditory meatus.
View Figure 4
Figure 4: MRI of Case 4 showing enhancement of facial nerve in internal auditory meatus.
View Figure 4
Perineural invasion (PNI) of the facial nerve has been estimated to occur in 10-30% of malignant parotid tumors, with adenoid cystic carcinoma showing the greatest propensity followed by adenocarcinoma not-otherwise-specified [3-5]. Cutaneous SCC is very common in NZ and Australia and, although it has a relatively lower rate of perineural spread of 2.5-14%, it is a more common cause of facial palsy in these countries than primary parotid malignancy. SCC is by far the most common head and neck malignancy and accounts for the greatest number of cases with involvement of cranial nerves [6,7].
Several factors have been associated with malignancy in the facial nerve. A study of 221 patients with tumor-related facial palsy showed that almost all had a gradual onset, which had been reiterated by multiple other authors [1,2,8,9]. Although sudden-onset facial paralysis is rarely due to an occult malignancy, it has been described to occur [10]. Sudden onset facial palsy is more common if the lesion is within the temporal bone, with an estimated rate of 20% [2]. The mean time of progression to complete paralysis is 8.7 months whilst the mean time to diagnosis varies from several months up to 5 years [2,9,11]. Although facial pain occurs in around 50% of cases with malignant involvement of facial palsy, the pain that occurs in Bell's palsy or Ramsay Hunt syndrome is usually present at the onset of the palsy and then improves [2,3]. If the pain persists it should be investigated, especially if the facial weakness persists.
Involvement of multiple cranial nerves should alert to a malignant process. It is estimated that 10-30% of PNI is clinically apparent, with the trigeminal nerve being most commonly affected, followed by the facial nerve [4,6]. Up to 40% of PNI is asymptomatic even when there is gross spread or imaging evidence [12,13]. Clinically apparent PNI of the trigeminal nerve portends a worse prognosis, and in our patients three of the four patients had facial numbness indicating trigeminal involvement (Table 1).
Table 1: Summary of patients' clinical journey. View Table 1
A previous history of head and neck skin cancer should alert to the possibility of a malignant cause as cutaneous SCC not infrequently involves the parotid gland [14]. While Case 3 had a cutaneous SCC on the ipsilateral side to his facial nerve palsy this was four years earlier, and he had an adenosquamous carcinoma. The other cases did not have a history of a previous cutaneous SCC. It is worth highlighting that most patients with cutaneous SCC will not get a facial palsy. Clinically evident PNI causing facial nerve palsy is more likely to occur in primary parotid malignancies with 33% as compared to from a metastasis, such as from cutaneous SCC to parotid, with 6% [5,7].
The prognosis for patients who have a malignancy with a facial palsy is much worse. A study of 103 patients with parotid malignancies, by Wierzbicka, reported a 3-year disease-free survival of 45% in those with facial nerve palsy vs. 88% those without [6]. Higgins reported a 5-year disease-specific survival of 19.1% with facial palsy vs. 65.2% without in a cohort of temporal bone and external auditory canal malignancies [15]. While in our series one patient has died from intracranial extension years following the onset of symptoms two others have incurable disease (Table 1).
It has been found that those with clinically evident PNI (CPNI) have a worse prognosis than those with pathological or incidental PNI (IPNI). Risk of locoregional recurrence in CPNI vs. IPNI is 37% vs. 17%, and the disease specific death is 27% vs. 6%. The 5-year recurrence free survival of CPNI vs. IPNI is 61% vs. 76%, and the disease specific survival is 70% vs. 88% [7]. The aggressiveness of the disease is reflected in our cases, with only two of four cases being treated with curative intent. Table 2 summarizes the survival of our patients, with one deceased 83 months following symptom onset.
Table 2: Summary of patients' survival. View Table 2
If a "Bell's palsy" does not behave as expected investigations should be performed to exclude alternative diagnoses. These include, but are not limited to, autoimmune blood work, identifying possible infectious sources depending on geography, and imaging. Although contrast-enhanced MRI is the modality of choice, false negative rates of 22-47% have been reported [6,9]. As such, a negative MRI does not preclude a malignancy and repeat imaging is often required. Similarly, non-targeted fine needle aspirate has a 40% false negative rate [6]. All our cases had an initial negative MRI workup and one of the two patients who had non targeted ultrasound guided parotid biopsies had a false negative result.
While there is reluctance to proceed to facial nerve exploration and biopsy to make a diagnosis this must be weighed against the consequences of a delay in the diagnosis of a malignancy. Despite multiple papers discussing this topic, there is no agreed algorithm. Patients continue to present to specialist services in a delayed manner with a diagnosis of "Bell's palsy" with investigations eventually showing malignancy. Unfortunately, for some, the disease has already progressed beyond what is treatable. For instance, two of our four patients had incurable disease at the time of diagnosis, with a median time to diagnosis of 14.5 months. While some authors recommend that a prolonged history of facial nerve palsy without recovery requires at least repeat imaging at about 6 months, this will result in a delay in diagnosis or even no diagnosis in some patients [1,2,8,10]. For instance, Case 3 had repeated MRIs which were negative even after the diagnosis was made, suggesting some malignancies (adenosquamous in this case) are notalways apparent on MRI, even after retrospective review by an experienced radiologist. It is recognized that those with higher risk features, such as pain, paresthesia, progressive pattern, and history of regional skin SCC, may require diagnostic exploration even with negative repeat imaging [2,3,8].
While it would be helpful to have figures for how many undiagnosed progressive facial palsies have an occult malignancy, as this would inform an algorithm, we are not aware these figures exist. Also helpful to know would be in how many patients with a progressive facial palsy due to an occult malignancy early diagnosis makes a worthwhile difference.
In Bell's palsy most of the recovery is within 3 weeks, with some further recovery by 3 months. If recovery is not seen within this timeframe, particularly by 6 months, it is unlikely to occur [16]. It may be inferred that a progressive facial palsy due to malignancy is unlikely to recover, therefore we propose a more proactive approach with repeat imaging at 3 months and, if there are high-risk features, to consider early operative exploration. In those without high-risk features, then consider exploration at 6 months, as there is unlikely to be facial nerve recovery by this point. There is a case for facial reanimation procedures to be done at the same time as the facial nerve exploration.
While occult malignancy causing facial nerve palsy is rare, it is not uncommon for the palsy to be diagnosed initially, and for some time thereafter, as Bell's palsy. This case series and literature review highlights the importance of a high index of suspicion, especially with a progressive facial palsy, even in absence of positive imaging. We recommend a more proactive approach whereby repeat imaging is performed at three months with operative exploration in patients with high-risk features, such as history of cutaneous SCC. In those without high-risk features a further period of observation may be undertaken.
This case report follows local research guidelines and does not require IRB approval. Patient information has been anonymised. Clinical photographs and imaging for inclusion in publication has been utilised with consent by patients (signed consents available upon request).
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
None to disclose for all authors.
None to disclose for all authors.
None.