Eustachian tube dysfunction is a common medical condition affecting approximately 1% of the adult population. Previous studies have evaluated eustachian balloon tuboplasty using tympanometry, ability to perform Valsalva maneuver and subjective improvement in symptoms to measure effectiveness. In this study, we propose using the Sino-Nasal Outcome Test (SNOT-22) to measure outcomes following eustachian tube balloon dilation, with a focus on ear-specific symptoms within the survey.
To evaluate ear-specific symptom outcomes following eustachian tube balloon dilation using the SNOT-22 questionnaire.
A retrospective chart review was performed on all patients who underwent eustachian tube balloon dilation from February 2015 to June 2017. Patients who completed the SNOT-22 survey before and after undergoing elective eustachian tube balloon were included. The mean difference of total scores as well as ear-specific symptom scores were compared. Paired T-tests were performed to evaluate whether there was a significant improvement in symptoms. Ear-specific scores included ear fullness/popping, ear pain, and dizziness.
17 patients completed pre-operative and post-operative SNOT-22 questionnaire. The mean pre-operative ear-specific SNOT-22 symptom score was 6.94. The post-operative score was 3.47, with a mean difference of 3.47 (95%CI: 1.05-5.89; p = 0.0032). The mean pre-operative total SNOT-22 score was 35, post-op score was 15.35 with a mean difference of 19.65, (95%CI: 8.33-30.97; p = 0.0006).
Patients have significant improvement in SNOT-22 ear-specific symptom scores following eustachian tube balloon dilation.
Eustachian tube, Balloon sinus dilation, Quality of life, Ear fullness, SNOT-22, Surgical outcomes, Sinus scoring system
Eustachian tube dysfunction (ETD) is a common medical condition affecting approximately 1% of the adult population [1]. It affects both children and adults, and may manifest as muffled hearing, aural fullness, pain, ear popping, tinnitus, or problems with balance. Long-term dysfunction may potentially lead to serious consequences including hearing loss, cholesteatoma, and chronic suppurative otitis media [2,3]. Unfortunately, no treatment has been shown to be consistently effective at improving eustachian tube function [4]. Furthermore, studies on pharmacologic management with nasal steroids, topical decongestants, and combined antihistamine and ephedrine have not demonstrated significant efficacy [5]. Direct surgical management of ETD using eustachian tube (ET) catheterization, bougie dilation, stenting, and ET obliteration has had limited success [6] and has been mostly abandoned due to safety concerns [7]. Some small cohort studies have demonstrated ETD improvement following removal of inflamed tissue with lasers and microdebriders [8-10], though the literature on these procedures is inconsistent [11].
Eustachian tube balloon dilation (ETBD) is an emerging potential solution for ETD. Most studies investigating the efficacy of ETBD have had small sample sizes and have used tympanometry [12-14], ability to perform a Valsalva maneuver [9,15,16], and subjective improvement in symptoms as outcome measures [12,17]. The results of these studies have been promising, with significant numbers of patients demonstrating improvement, and little to no serious complications [12,18,19]. Recently, several studies have demonstrated significant improvements following ETBD using the Eustachian Tube Dysfunction Questionaire-7 (ETDQ-7) [3,20,21], a disease specific assessment for symptoms of eustachian tube obstruction [22].
In this study, we propose using the Sino-Nasal Outcome Test (SNOT-22) to measure outcomes following eustachian tube balloon dilation. The SNOT-22 is a validated measure of sinonasal-specific symptoms and quality of life [23]. We chose the SNOT-22 to evaluate ETDB because of the frequent comorbidity of ETD and sinonasal disease [21] and the SNOT-22's inclusion of quality of life measures. Only one study has previously analyzed balloon dilation using the SNOT-22 where the majority of patients concurrently underwent balloon dilation and endoscopic sinus surgery [21]. Using the SNOT-22, this present study examines the efficacy of ETBD and its impact on related symptoms and quality of life.
This study was approved by the Northwell Health System Institutional Review Board. A retrospective review of previously prospectively collected data was performed. Data was collected from February 2015 to November 2017 at a single institution of patients undergoing eustachian balloon dilation for ETD. While the information was collected prospectively, its original purpose was for clinical practice improvement. Patients who did not fill out pre-operative (pre-op) or post-operative (post-op) SNOT-22 questionnaires were excluded. Pre-op and post-op SNOT-22 scores were recorded, as well as ear-specific SNOT-22 scores (ear pain, ear fullness/popping, dizziness). Data analysis was conducted using STATA 15. A Paired t-test was performed to determine statistical significance between pre-op and post-op SNOT-22 scores. A p-value of less than 0.05 was deemed statistically significant.
In this series, eustachian tube ballooning occurred during functional endoscopic sinus surgery. In all cases, a CT scan of the sinuses was reviewed preoperatively to evaluate for any eustachian tube anomalies or additional pathology that could otherwise explain the patients' symptoms. After the endoscopic sinus portion of the surgery was complete, the nasopharynx was visualized with a 0-degree endoscope. The eustachian tube was identified. The Acclarent Balloon Dilation System (Acclarent, Inc; Irvine, CA; 2018) was used for the ETBD portion of the surgery. The frontal guide on the Acclarent device was used to access the eustachian tube orifice and advanced to the yellow marker (Figure 1). The tip was directed laterally and in the 10:00 position on the right and the 2:00 position on the left. If any resistance was met or there were any signs of bleeding the balloon was not advanced any further. Once placed in the correct position, the balloon was inflated to a pressure of 10 and held for two minutes duration, and then removed. After removing the balloon, attention was made to suction cauterize any adenoidal hypertrophy or any synechiae from prior adenoidal surgery. Of note, this was an off-label use of the frontal guide and the wire portion of the device.
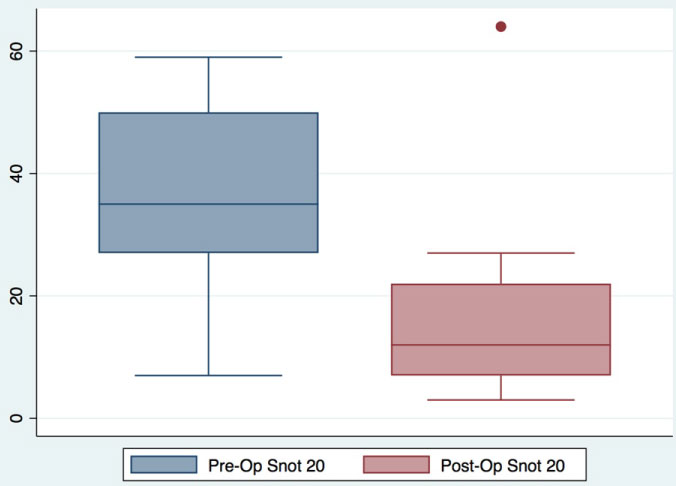 Figure 1: Pre and postoperative SNOT-22 scores. Preoperative total SNOT-22 mean score was 35, post-op score was 15.35, mean diff 19.65 95CI (8.33-30.97) p = 0.0006.
View Figure 1
Figure 1: Pre and postoperative SNOT-22 scores. Preoperative total SNOT-22 mean score was 35, post-op score was 15.35, mean diff 19.65 95CI (8.33-30.97) p = 0.0006.
View Figure 1
Thirty-three patients underwent eustachian balloon dilation during the study period. Of those, seventeen completed both pre-op and post-op SNOT-22 questionnaires. Four patients did not complete either the pre-op or post-op questionnaire. Twelve patients only completed a preop questionnaire. Sixty-two percent of patients were male and the average age was 47-years-old. All but two patients had a decrease in overall, as well as ear-specific symptom, SNOT-22 scores. The change in SNOT-22 scores ranged from +5 to -45. Similarly, the change in ear-specific symptom scoring ranged from +2 to -10. For those who completed both the pre-op and post-op questionnaires, the mean pre-op total SNOT-22 score was 35 and the post-op score was 15.35, with a mean difference if 19.65 (95%CI: 8.33-30.97; p = 0.0006) (Figure 2). Similarly, the mean pre-op ear-specific symptom SNOT-22 score was 6.94 and the post-op score was 3.47, with a mean difference of 3.47 (95%CI: 1.05-5.89; p = 0.0032) (Figure 3). Even when those who did not fill out post-operative questionnaires were included for review, a statistically significant decrease in SNOT-22 score between groups before and after surgery was observed.
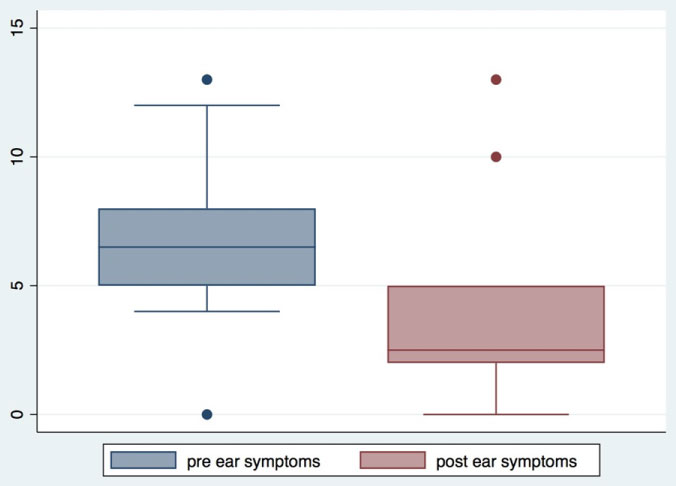 Figure 2: The mean pre-operative score of ear specific SNOT-22 symptoms was 6.94, post-op score was 3.47 with a mean diff 3.47 95CI (1.05-5.89) p = 0.0032.
View Figure 2
Figure 2: The mean pre-operative score of ear specific SNOT-22 symptoms was 6.94, post-op score was 3.47 with a mean diff 3.47 95CI (1.05-5.89) p = 0.0032.
View Figure 2
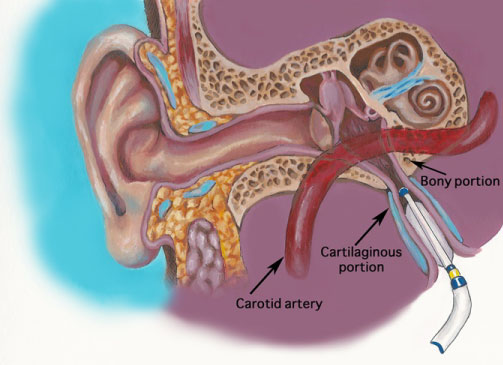 Figure 3: This figure demonstrates the technique used to dilate the Eustachian Tube. The frontal guide on the Acclarent device is used to access the Eustachian tube orifice and advanced to the yellow marker.
View Figure 3
Figure 3: This figure demonstrates the technique used to dilate the Eustachian Tube. The frontal guide on the Acclarent device is used to access the Eustachian tube orifice and advanced to the yellow marker.
View Figure 3
The eustachian tube (ET) is a complex osseocartilaginous connection between the protympanum and the nasopharynx. The ET acts to protect the middle ear from sources of disease, to ventilate the middle ear, and to help drain secretions away from the middle ear. Eustachian tube dysfunction (ETD) is the inability of the ET to adequately perform these functions. The precise function and mechanisms of the ET and the underlying causes of dysfunction are complex and not fully understood [4]. Despite recent proposed guidelines, there is currently no universally accepted diagnostic criteria and definition for ETD [24]. However, it is still associated with widespread health care utilization, especially in primary care and otolaryngology settings and is estimated to account for over 8 million dollars in health care costs annually [25]. Our limited understanding of the epidemiology of ETD in adults makes it difficult to define optimal treatment options for these patients [2].
First described in 2010, eustachian tube balloon dilation (ETBD) is an innovative approach designed to treat the underlying cause of ETD. This minimally invasive intervention aims to dilate and open the cartilaginous part of the eustachian tube [26]. A recent systematic review by Luukkainen, et al. suggests promising long-term results for balloon eustachian tuboplasty (BET) in regards to improvement in overall subjective symptoms, Valsalva maneuver, and otoscopic findings. Results using tympanometry and tubomanometry were less encouraging [27]. However, the relatively small study populations, short follow-up times, as well as significant heterogeneity of the studies regarding indications, follow-up duration, and outcome measures makes any direct comparisons and definitive conclusions difficult to ascertain [26].
In this study, patients reported significant symptoms consistent with ETD, along with rhinologic complaints during their pre-operative office encounters. Therefore, treatment with balloon dilation (an off-label use of the Acclarent balloon device) was offered at the time of endoscopic sinus surgery to address these symptoms. Nearly all patients noted a statistically significant benefit from ETBD in terms of both overall, as well as ear-specific symptom SNOT-22 scores, respectively. The authors believe that addressing the concomitant sinus disease may be important in this type of patient population as the sinus disease may be contributing to the ETD. Our results are similar to that of McCoul, et al., who also showed significant improvement in SNOT-22 scores following ETBD [21].
In this series, SNOT-22 questionnaire scores were used as the primary outcome measure. It is standard in the senior author's (L.L.) practice to provide the questionnaire to all patients who report any symptoms with potential sinus etiology. The SNOT-22 is a patient reported, disease-specific quality of life outcome measures in sinonasal disorders. It has demonstrated strong reliability, validity and responsiveness, and has even been translated and revalidated in multiple languages [23]. It is also commonly used in ENT offices and incorporates global quality of life metrics. While newer attempts to subjectively quantify ETD have been developed using the seven-item Eustachian Tube Dysfunction Questionnaire ('ETDQ-7'), it does not appear to be adequate in addressing those patients with a patulous eustachian tube or those with concomitant sinonasal disease, such as our patient population. Additionally, several studies have shown good correlation between ETDQ-7 and SNOT-22 scores in regards to ETD symptoms [21,28,29]. It is for this reason, this study chose to look at SNOT-22 scores for overall improvement, as well as ear specific scores to provide a more holistic view of the disease processes facings these patients- as the majority presented with a constellation of symptoms, not only ETD.
As with all retrospective reviews, this study is limited by its design. The lack of a control group demonstrates the need for further trials on the subject. The concomitant endoscopic sinus surgery may present a confounding variable that explains post-operative improvement. Additionally, the optimal measure of treatment success following ETBD has not been determined. Previous studies have utilized various outcome measures making it difficult to compare our results to that of prior studies. It is important to note that none of our patients had concomitant myringotomy and tympanostomy tube placement procedures, which in hindsight, may be useful in some cases. Furthermore, differentiating underlying ETD as a result of patulous eustachian tube versus functional congestion/obstruction should be done in this patient population to prevent unnecessary dilation and potentially worsening the symptoms.
In this study, patients had significant improvement in ear-specific symptoms after eustachian tube balloon dilation and adds to the growing evidence of potential quality of life benefits derived from the procedure. Eustachian tube balloon dilation offers a potential opportunity to treat a poorly understood disease process that can significantly impacts a patient's quality of life.
None.
The authors have no conflicts of interest to disclose.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors have no conflicts of interest or funding to disclose. All authors had equal contribution to execution of this research.