Bausch Health Canada cardiovascular and metabolic treatment portfolio has been shown to be safe and effective in clinical trials. However, in clinical trials, data is collected in selective populations under ideal conditions, making inference to the real-world problematic. Well executed patient registries are required to provide evidence of the benefits of therapeutic agents under routine, real world conditions. The 'Cardio-Vascular and metabolic treatments in Canada: Assessment of REal-life therapeutic value' registry (CV-CARE) is the first attempt to describe the real-world effectiveness of Bausch Health Canada cardiovascular and metabolic treatments. Our objectives are to introduce CV-CARE and describe baseline patient, disease and treatment characteristics.
CV-CARE was a Canadian, multi-center, community-based, prospective cohort registry in patients initiating one or more of Bausch Health Canada cardiovascular and metabolic treatments; metformin extended-release for type 2 diabetes mellitus (T2DM), colesevelam for hypercholesterolemia (HCh), and/or azilsartan (AZI), azilsartan/chlorthalidone (AZI/CHL), or diltiazem extended-release (TXC) for hypertension (HTN). CV-CARE ran in 300 specialist and GP centers between 2015-2018. Treating physicians used standardized case report forms, patient interviews and chart reviews for data collection. Descriptive statistics were used to describe baseline patient, disease and treatment characteristics.
The registry enrolled 6960 patients; 4194 patients make up this baseline population. 56.3% were male, 50% were Caucasian. Mean age was 59.6 years. 23.7% were treated for T2DM, 39% for HCh, and 49.1% for HTN. Mean disease duration was 4.9 years (T2DM), 4.9 years (HCh) and 6.7 years (HTN). Mean body mass index was 30 kg.m-2. 11% were current smokers; 80% previous smokers.
This paper describes, for the first time, the CV-CARE registry and characterizes baseline demographics and disease/treatment characteristics of Canadian patients on a Bausch Health Canada cardiovascular and metabolic treatment. The CV-CARE registry will be utilized to assess the real-world effectiveness of Bausch Health Canada treatments, and to investigate regional variability of disease management across Canadian provinces.
CV-CARE registry, Hypercholesterolemia, Hypertension, Routine-care, Type 2 diabetes mellitus
A1C: Glycated hemoglobin; A: Albumin creatinine ratio; B: Body mass index; DBP: Diastolic blood pressure; Egfr: Estimated glomerular filtration rate; FPG: Fasting plasma glucose; HDL-C: High density lipoprotein cholesterol; LDL-C: Low-density lipoprotein-cholesterol; SBP: Systolic blood pressure; TC: Total cholesterol; TG: Triglycerides
Cardiometabolic disease is a collection of metabolic dysfunctions categorized by overweight or obesity, insulin resistance/dysglycemia, dyslipidemia and hypertension [1,2]. In Canada, 2.4 million people have been diagnosed with diabetes, 90% of which have type 2 diabetes mellitus (T2DM) [3]. Glycated hemoglobin (A1C) reductions are linked to reduced risk of diabetic complications [4]. However, only 50% of patients with T2DM in Canada meet the A1C target of ≤ 7.0%, only 57% reach the low-density lipoprotein-cholesterol (LDL-C) target of ≤ 2.0 mmol/L, only 36% reach the systolic blood pressure (SBP) < 130 mmHg, and only 13% reach the triple target [5]. Metformin immediate-release (IR) is a traditional first-line antihyperglycemic agent but up to 25% of metformin IR patients develop gastrointestinal (GI) side effects leading to cessation of use [6]. Metformin extended-release (MetER) preparations may be associated with fewer GI side effects compared to metformin IR [7]. A once daily MetER was developed for better adherence, with efficacy and safety results similar to metformin IR [8]. It was approved for use by Health Canada in 2011 [9].
Hypercholesterolemia (HCh) is directly associated with an increased risk of cardiovascular disease [10-12]. Data from clinical trials on more than 170,000 patients evaluating lipid-lowering medications has shown that lowering LDL-C is associated with significant reductions in cardiovascular morbidity and mortality [13]. As a result, the Canadian Cardiovascular Society recommends a primary target of lipid-lowering therapy (LLT) of LDL-C to < 2.0 mmol/L and/or by 50% [14]. In high risk patients, an LDL-C target of 1.8 mmol/L should be considered. This reduction considerably lowers the risk of cardiovascular disease, mortality, nonfatal myocardial infarction, and recurrent cardiovascular disease events [15,16]. Additional reductions in LDL-C to 1.4 mmol/L and even below 1 mmol/L in very high-risk patients are associated with further cardiovascular event reduction [17,18] and are recommended by the 2019 European Society of Cardiology lipid guidelines [19]. Many patients on statins, the standard of care for hypercholesterolemia, do achieve therapeutic goals [20]. For those not achieving target, the Canadian Cardiovascular Society recommends add-on therapy (ezetimibe, bile acid sequestrants, PCSK9 inhibitors) for high-risk patients [14]. The bile acid sequestrant Colesevelam (C) hydrochloride (HCL) was approved by Health Canada in 2011 for the reduction of blood cholesterol in patients with hypercholesterolemia based on studies that showed greater reductions in LDL-C compared to placebo at all doses studied [21].
Elevated blood pressure is the leading risk factor for death and disability-adjusted life-years lost [22]. The Canadian prevalence of hypertension (HTN) is estimated at 20% and is forecasted to increase [23]. Despite the availability of a variety of anti-hypertensive treatments in Canada, 34% of treated patients are still not at target [24]. In 2014, azilsartan medoxomil (AZI) [25] an angiotensin receptor blocker (ARB), was launched in Canada for the treatment of mild to moderate essential HTN [26,27]. In 2016, Health Canada also approved a fixed dose combination of azilsartan medoxomil with chlorthalidone (AZI/CHL) [25] for the treatment of severe HTN [28,29]. Both new treatments were shown to significantly decrease blood pressure compared to best in class standard of care [27,28]. Diltiazem extended-release (ER) [30], a calcium channel blocker (CCB) for the treatment of mild to moderate essential HTN, provides an additional medication choice with a novel delivery system proven to be effective in 24 hr blood pressure control [31].
Metformin ER, C, AZI, AZI/CHL and Diltiazem are part of the bausch health canada (BHC) cardiovascular (CV) and metabolic (MET) portfolio. Although all drugs have been shown to be safe and effective in clinical trials, such data is collected in selective populations under ideal conditions, making inference to the real-world problematic [32]. Post approval clinical epidemiological studies (PACES), including post marketing prospective observational studies and patient registries, are the only source of information that allows assessment of real-world effectiveness [32]. The CV-CARE registry is the first systematic attempt to assess patients being treated with BHC CV/MET treatments in Canada in routine clinical care settings.
The primary objective of the CV-CARE registry was to describe the real-world effectiveness of CV/MET treatments in BHC portfolio (MetER for T2DM, C for HCh and AZI, AZI/CHL or TXC for HTN), in patients with CV/MET diseases followed under Canadian routine clinical practice. Secondary objectives of CV-CARE were to describe the profile of patients treated with the registry drugs and to investigate the regional variations across Canadian provinces. This report describes the CV-CARE registry for the first time and the baseline profile of patients.
The registry was a multi-site, Canadian, community-based, prospective, non-interventional program. Three hundred sites across Canada were recruited. Patients were recruited by treating physicians or nurse practitioners at each site. There were no interventions in patient management and treatments used were those as per the standard of care at each site and the clinical judgement of the treating physician. All patients were followed for 24 months and underwent three visits; day 0 (baseline), month 12 (± 6 months) and month 24 (± 6 months). Study assessments and administration of a case report form (CRF) questionnaire took place during routine care at each site. The decision to treat patients with study medication(s) must have been reached prior to, and independent of, the patient being enrolled into the registry. The study was approved by independent, centralized research ethics committees and informed consents were obtained. An independent clinical research organization (JSS Medical Research) designed and executed the registry on behalf of the sponsor (Valeant Canada).
Between 2015-2018 consenting patients who had a diagnosis with any combination of T2DM, HCh, and/or HTN and who were prescribed one or more of the study drugs as part of their routine clinical care, were entered in the registry. Patients were eligible for CV-CARE registry entry if they were treated with a study drug(s), were ≥ 18 years of age, the treating physician had reached a decision to treat the patient with the studied drug(s) prior to, and independently of, soliciting the patient to participate in the study, and the patient or legal representative (when allowable by law) had provided written informed consent. Patients were excluded from the registry if they had any contraindications to the use of study drug(s) as specified in the Canadian Product Monographs, and/or if the patient had any condition which, as per the judgment of the treating physician, prohibited them from participating in the study.
All patients were treated and followed for 24 months. The scheduling of procedures is outlined in (Table 1 and Table 2).
Table 1: Study assessments as per local practice. View Table 1
Table 2: Disease parameters as per local practice. View Table 2
Patient's demographics (date of birth, sex, smoking status (Y/N), # cigarettes per day, previous smoking duration and pack years (pack years = # cigarettes/day x # years as a smoker) and race (Caucasian, Black, Asian, Hispanic and Other)) were collected via patient interviews. Height (m), weight (kg) and waist circumference (cms) were measured in the treating physician's office according to local standards. Date of disease diagnoses, medical history, including comorbid cardiovascular/metabolic diseases were collected via patient interview and chart review.
Disease parameters were evaluated at baseline, month 12 and month 24. Blood and urine samples were collected and sent for laboratory testing as per local clinical practice. Pulse, SBP and diastolic blood pressure (DBP) were measured at the physicians' office following the 2013 canadian hypertension education program (CHEP) guidelines [33]. The average of the 2 measurements was used for the evaluation of blood pressure control. The 2013 clinical practice guidelines for the pharmacologic management of T2DM [34], the 2012 Canadian cardiovascular society guidelines for the diagnosis and treatment of dyslipidemia in adults [35], and the 2015 hypertension education program recommendations for blood pressure and treatment of hypertension [36] were used to diagnose T2DM, HCh and HTN respectively. Dose of study drugs were assessed at baseline and subsequent dose changes, along with associated reasons, were recorded at each follow up visit (month 12 and month 24). All concomitant medications used and any changes over time were documented, including dose, frequency and duration of treatment. All adverse events (AEs) and serious adverse events (SAEs), including major cardiac events (MACE) were reported to the sponsor pharmacovigilance department.
The decision to initiate treatment was solely the responsibility of the treating physician and the patient. As such, it was a non-randomised process. Given the observational nature of the registry, all treatments were accessed as per routine clinical practice in each region. More specifically, the sponsor, the study coordination centre or investigators did not supply or reimburse for any treatment use by the patients in the study. Patients were prescribed the medications by the treating physician and acquired medications through available insurance plans/government reimbursement or purchase with their own funds.
As CV-CARE was a real world, observational study with the aim to collect data during routine, Canadian clinical care, all measurements defined in the protocol were requested. However, investigators were asked to conduct their clinical practice as they would in 'real life' and collect data as per their routine clinical care. As a result, data gaps were expected. To maintain the observational nature of the study, violations of inclusion and exclusion criteria did not necessarily result in discontinuation of the patients. Patients were maintained in the study if, in the opinion of their physician, treatment with the studied drug(s) continued to be indicated and was safe.
The primary outcome of the registry was to assess real-life effectiveness of MetER, C, AZI, AZI/CHL and Diltiazem. Specific disease primary outcomes were; 1) For patients with T2DM treated with MetER the absolute change in baseline A1C and fasting plasma glucose at 12 ± 6 months, 2) For patients with HCh, the percent change from baseline LDL-C at 12 ± 6 months, 3) For patients with HTN, the absolute change from baseline SBP at 12 ± 6 months. Secondary outcomes for the registry included the primary outcomes mentioned above at 24 ± 6 months and, in addition, the following; for patients with HCh, the absolute change in total cholesterol, HDL-C, Apo-B, non-HDL-C, triglycerides, A1C and fasting plasma glucose at 12 ± 6 months and 24 ± 6 months; for patients with HTN, the achievement of target BP (rate of responders) as per CHEP [36]; proportion of patients still on study drugs at 1 and 2 years; and safety and tolerability of study drugs assessed by SAEs, including MACE and treatment emergent AEs. Sample size determination was based on known clinically meaningful differences for our chosen primary outcome variables and a conservative estimate of expected changes over 12 months. Precision was assessed using 95% confidence intervals. A minimum of 1945 patients treated with MetER were required to obtain sufficient precision with respect to A1C reduction; 2751 patients treated with C were required to obtain sufficient precision with respect to LDL-C reduction; 480/949 patients were required in order to obtain sufficient precision for changes in SBP and DBP with AZI respectively; and 1362 (AZI/CHL) patients were required to obtain sufficient precision for changes in SBP. Assuming a 10% attrition rate by 12 months, a sample size of 8000 patients were deemed required.
For this paper, we aimed to characterize the baseline demographics and disease/treatment characteristics of Canadian patients on a BHC CV/MET treatment. To that aim, descriptive statistics, including mean and standard deviation (SD) for continuous variables and counts and proportions for categorical variables, were used to describe the baseline patient profile and treatment. Two patient cohorts were defined for analysis - primary analysis population and safety population. The primary analysis population comprised all patients enrolled in the registry, who had received at least one dose of study drug, had a baseline visit and at least one follow up assessment visit, and met the selection criteria. The safety population were all those patients that received at least one dose of study drug. Given the observational nature of the study, all observations were included in the analyses and no data imputation for missing data took place. Histogram and formal tests for distribution found the baseline data to be normally distributed. All analyses were performed using SAS (version 9.4, SAS Institute Inc, Cary, NC) or SPSS (version 21.0, IBM Corp, Armonk, NY) software.
In July 2018, following a thorough analysis of the datasets, it became clear that the primary outcomes had been met. As such, the decision was made to stop the registry early and study enrollment was closed in August 2018 with a final sample size of 6960 patients (Figure 1). Baseline characteristics, stratified by study drug, are shown in Table 3. Overall, 995 patients were prescribed MetER, 1639 C, 1364 AZI, 498 AZI/CHL, and 199 Diltiazem. Mean age was 59.6 ± 11.7 years, patients were predominately male (56.3%), Caucasian (50.0%), with 22% being overweight (BMI > 27 mg.kg2) and 42% having obesity (> 30 mg.kg2). Approximately 11% were current smokers, while 80% reported being previous smokers. The average disease duration was 4.9 years (T2DM), 4.9 years (HCh) and 6.7 years (HTN), with 23.7% being treated for T2DM, 39% for HCh and 49.1% for HTN.
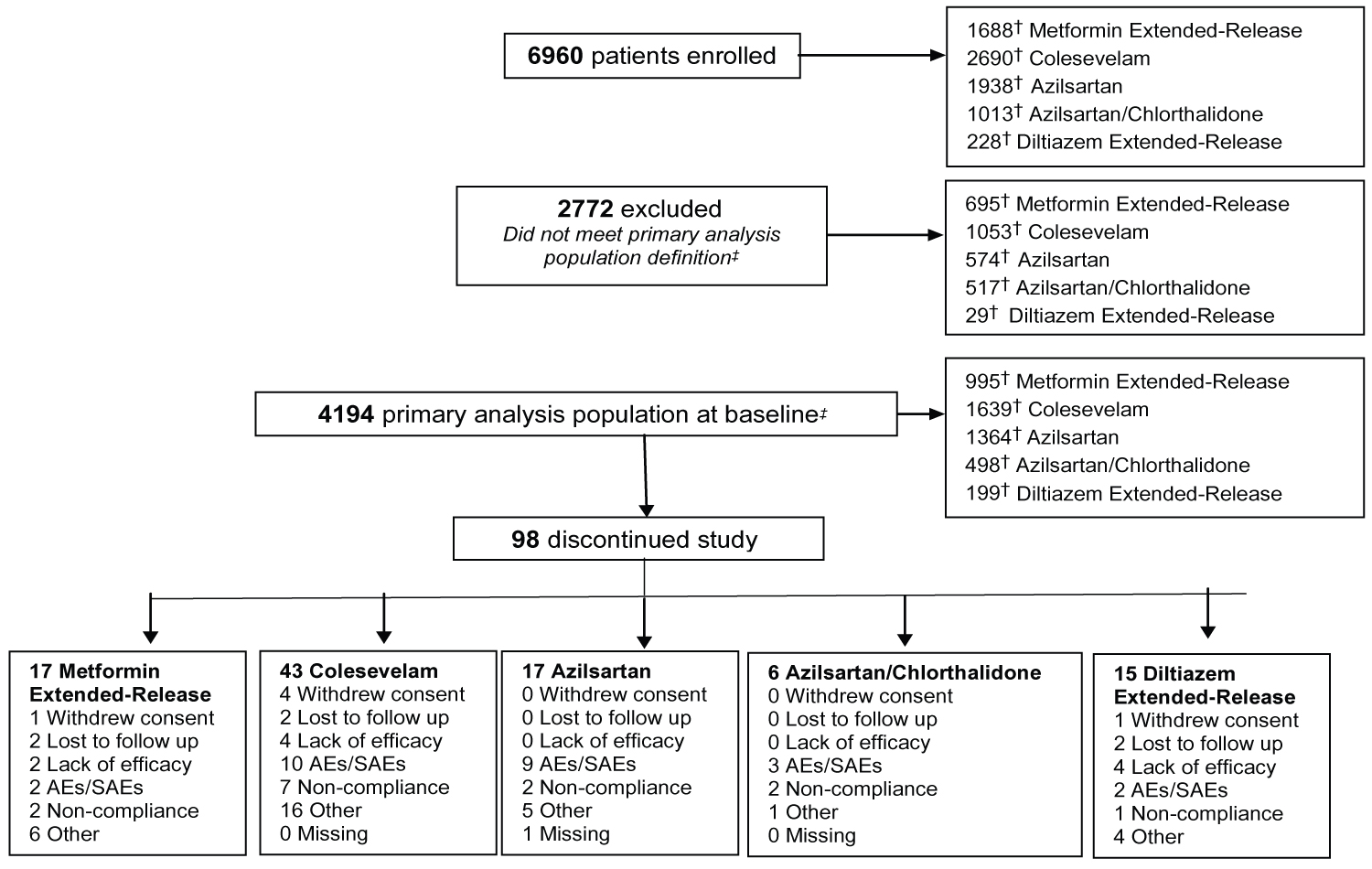 Figure 1: Flow of patients through the CV-CARE registry.
Figure 1: Flow of patients through the CV-CARE registry.
†Note that patients may have taken more than one study drug and therefore maybe represented in more than one study drug group; ‡Defined as all enrolled patients in the study who received at least one dose of study drugs, had at least one follow-up assessment visit and meet the selection criteria.
View Figure 1
Table 3: Patient demographics and disease characteristics by study drug†. View Table 3
The diagnosis for initiating treatment with MetER was primarily T2DM (98.8%). There were 3121 concomitant conditions in 1276 (75.5%) of the MetER patients, with the majority of conditions classified as metabolic and nutritional disorders (57.9%), followed by vascular disorders (53.9%), and cardiac disorders (5.7%) (Figure 2A). There were 4413 concomitant medications being taken in 1427 (84.4%) of the MetER patients, with antihyperglycemic agents (AHAs), LLT, and renin-angiotensin agents being the most common (Figure 3A). The most common total daily dose for MetER was 1000 mg (Figure 4A). Metformin was most often taken as a switch from Metformin IR (60.6%) versus being started as an initial treatment (26.5%) or use with other AHAs (9.8%). The major reason for switching from another treatment was tolerability (44.4%) (Figure 5A).
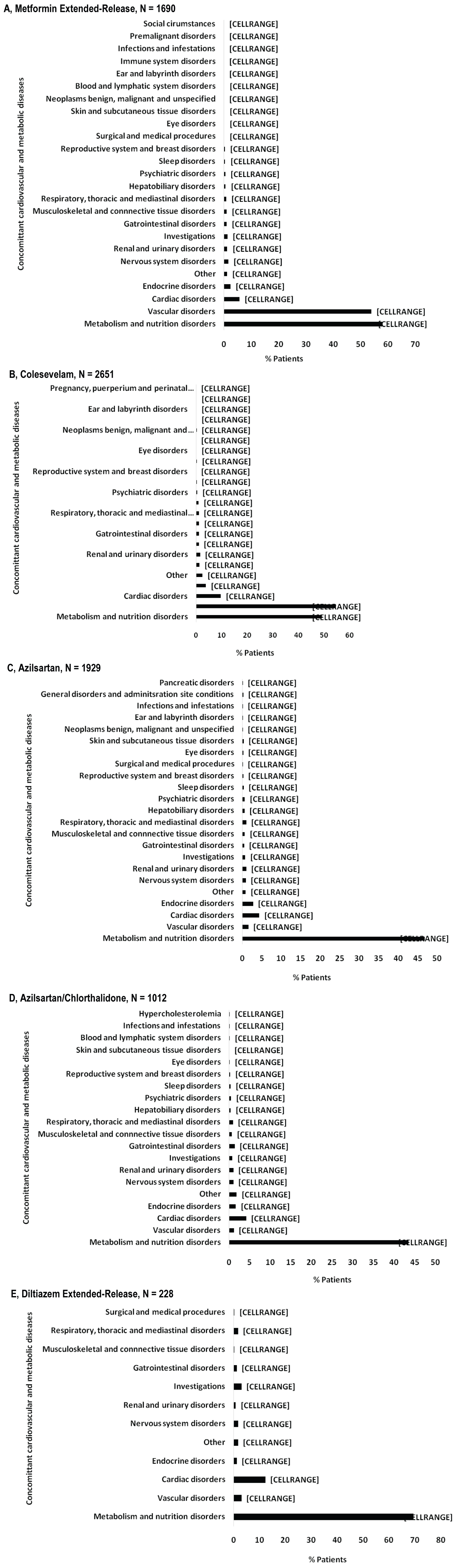 Figure 2: Concomitant cardiovascular and metabolic diseases by study drug. A) Metformin extended-release; B) Colesevelam; C) Azilsartan; D) Azilsartan/Chlorthalidone; E) Diltiazem extended-release.
Figure 2: Concomitant cardiovascular and metabolic diseases by study drug. A) Metformin extended-release; B) Colesevelam; C) Azilsartan; D) Azilsartan/Chlorthalidone; E) Diltiazem extended-release.
Note: Concomitant conditions reported as system organ class using the safety population.
View Figure 2
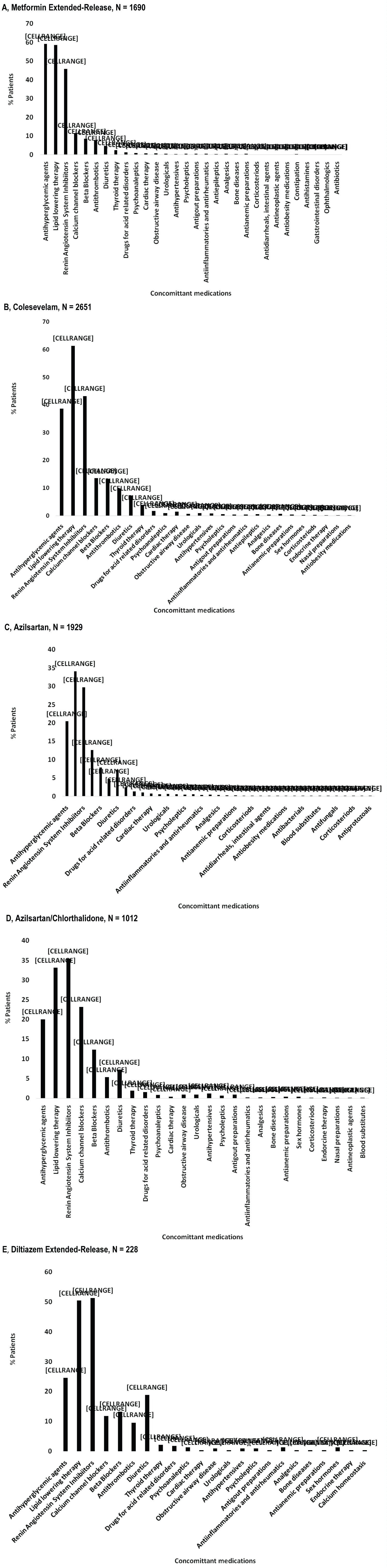 Figure 3: Concomitant medications by study drug. A) Metformin Extended-Release; B) Colesevelam; C) Azilsartan; D) Azilsartan/Chlorthalidone; E) Diltiazem Extended-Release.
Figure 3: Concomitant medications by study drug. A) Metformin Extended-Release; B) Colesevelam; C) Azilsartan; D) Azilsartan/Chlorthalidone; E) Diltiazem Extended-Release.
Note: Concomitant medications are reported at ATC level 2, using the safety population.
View Figure 3
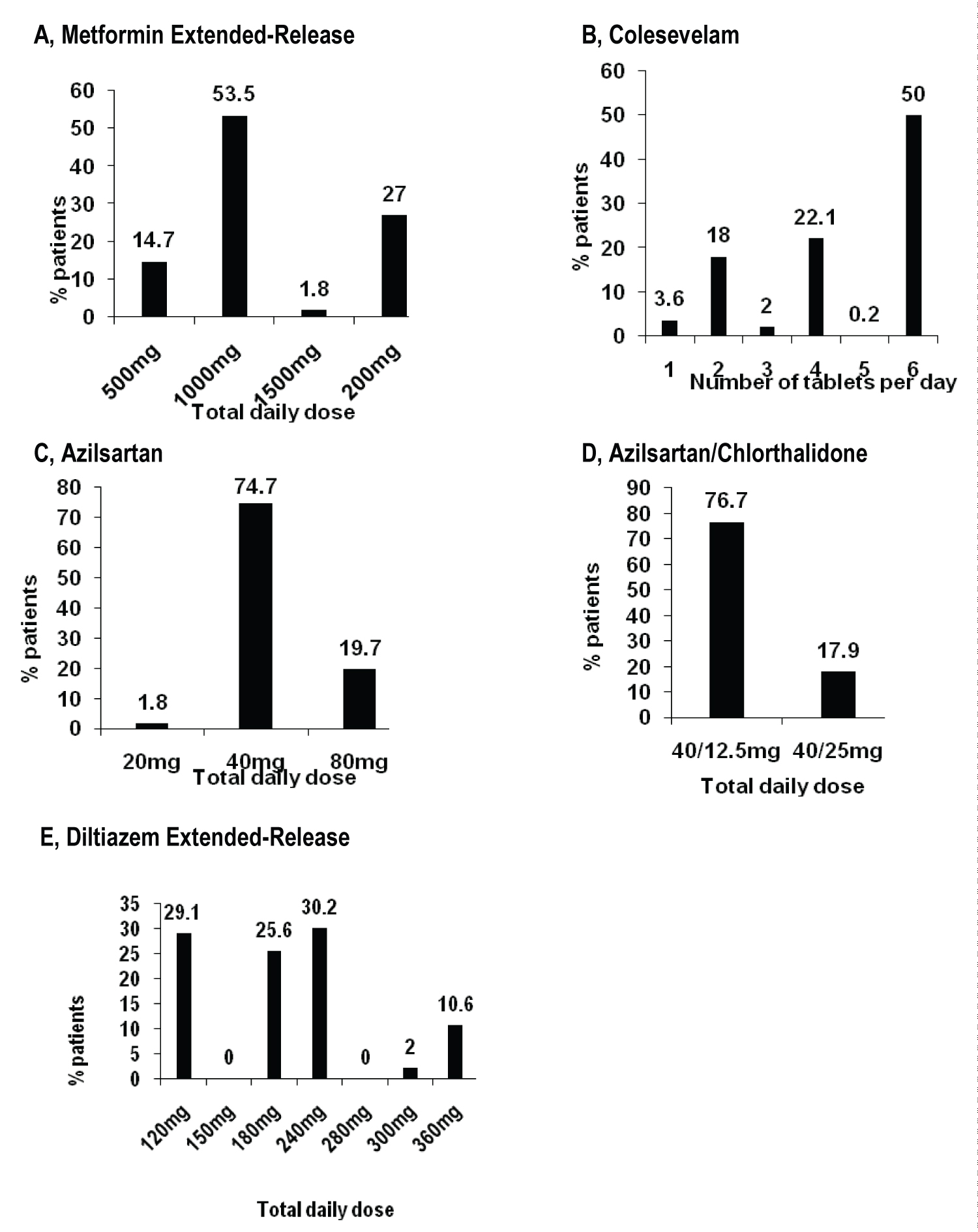 Figure 4: Treatment by study drug in primary analysis population. A) Metformin extended-release; B) Colesevelam; C) Azilsartan; D) Azilsartan/Chlorthalidone; E) Diltiazem extended-release.
View Figure 4
Figure 4: Treatment by study drug in primary analysis population. A) Metformin extended-release; B) Colesevelam; C) Azilsartan; D) Azilsartan/Chlorthalidone; E) Diltiazem extended-release.
View Figure 4
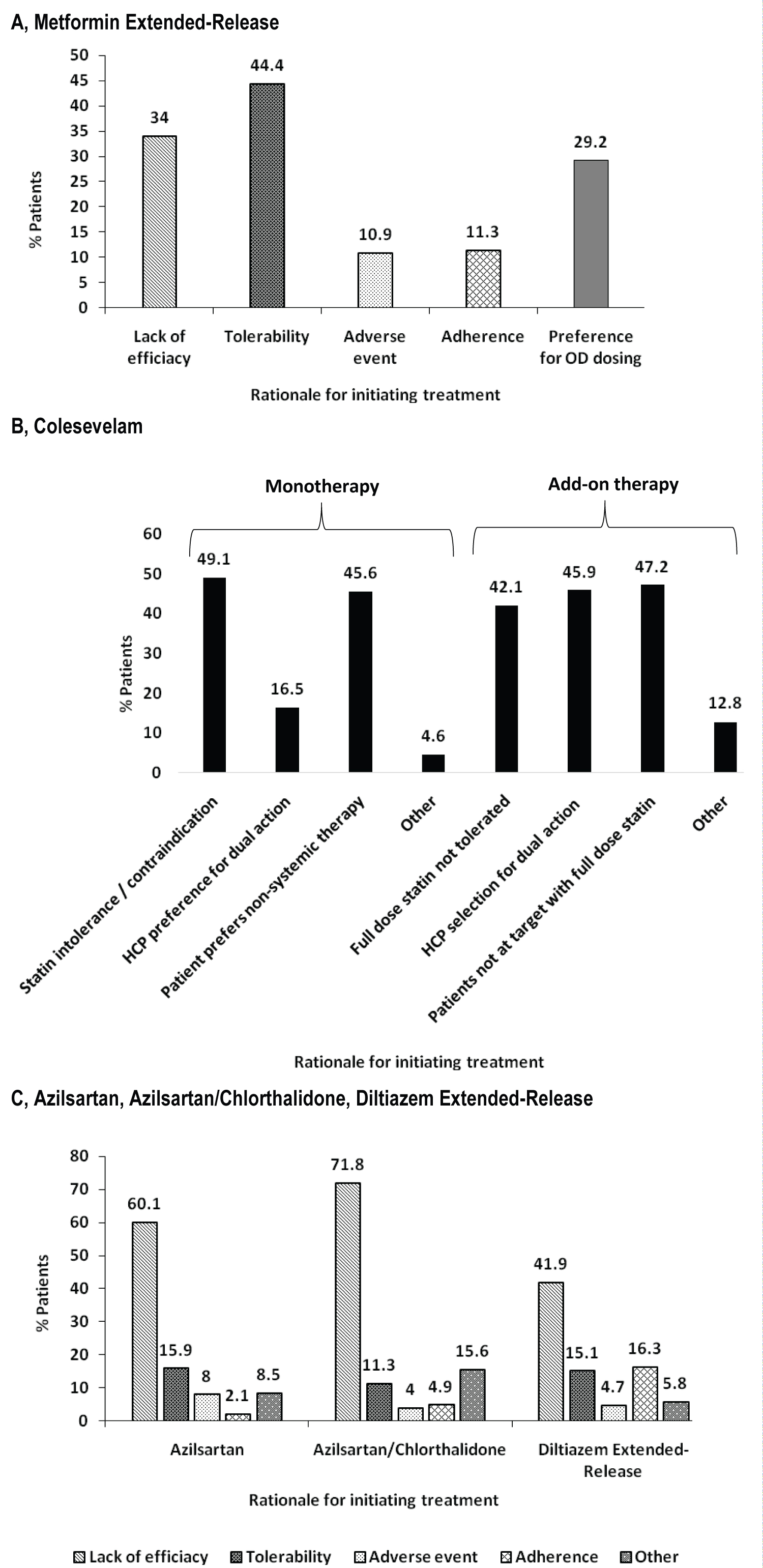 Figure 5: Rationale for initiating. A) Metformin extended-release; B) Colesevelam; C) Azilsartan, Azilsartan/Chlorthalidone, or Diltiazem extended-release.
View Figure 5
Figure 5: Rationale for initiating. A) Metformin extended-release; B) Colesevelam; C) Azilsartan, Azilsartan/Chlorthalidone, or Diltiazem extended-release.
View Figure 5
The diagnosis for initiating treatment with C was primarily HCh (95.9%). There were 4911 concomitant conditions in 2037 (76.8%) of C patients, with most of the conditions classified as vascular disorders (54.7%), followed by metabolic and nutritional disorders (49.2%), cardiac disorders (9.7%) and endocrine disorders (3.9%) (Figure 2B). There were 6777 concomitant medications being taken in the 2146 C patients (81%), with LLT, AHAs and renin-angiotensin agents being the most common (Figure 3B). Colesevelam was given in sachet powder form in 60.1% of patients, with 37.3% given daily tablets. Most patients taking tablets were prescribed 6 tablets per day (Figure 4B). Patients were given C as monotherapy (41.5%) or add-on therapy (57.1%). The major reason for monotherapy was statin intolerance or contraindication (49.1%); while the major reason for add-on therapy was patients not at target with full dose statin (47.2%) (Figure 5B).
The diagnosis for initiating treatment with AZI, AZI/CHL or TXC was primarily HTN (96.8%, 96.4%, 87.9% respectively). There were 1982 concomitant conditions in 993 (51.5%) of the AZI patients; 986 concomitant conditions in 494 (48.8%) of the AZI/CHL patients; and 305 concomitant conditions in 179 (78.5%) of the TXC patients. Most conditions were classified as metabolic and nutritional disorders (53.1%), followed by cardiac disorders (6.9%) (Figure 2C, Figure 2D and Figure 2E). There were 2880 concomitant medications being taken in 1260 (65.3%) of the AZI patients; 1768 in 672 (66.4%) of the AZI/CHL patients; and 524 in 196 (86%) of the TXC patients (Figure 3C, Figure 3D and Figure 3E). LLT, AHAs and renin-angiotensin agents were the most common concomitant medications (Figure 3C, Figure 3D and Figure 3E). The most common total daily dosing was 40 mg, 40/12.5 mg and 240 mg for AZI, AZI/CHL and TXC respectively (Figure 4C, Figure 4D and Figure 4E). For all hypertension drugs, treatment was generally initiated as a switch from prior treatment (AZI = 49.8%; AZI/CHL = 65.5%; TXC = 43.2%), versus initial treatment (AZI = 34.5%; AZI/CHL = 19.7; TXC = 19.6%) or add-on to a current therapy (AZI = 14.1%; AZI/CHL = 12.9%; TXC 37.2%). The most common medications that patients were switched from were angiotensin-converting enzyme (ACE) inhibitors (AZI = 49.8%; followed by ARBs 33.3%, diuretics 10.5%, CCBs 9.4%, beta blockers 7.2% and alpha blockers 2.5%), ARBs (AZI/CHL = 43.9%, followed by ACE inhibitors 42%, diuretics 35%, CCBs 13.8%, beta blockers 4.3% and alpha blockers 2.1%) or CCBs (TXC = 67.4%, followed by beta blockers 14%, ACE inhibitors 9.3%, diuretics 8.1% and ARBs 1.2%). The major reason for switching was lack of efficacy (AZI = 60.1%; AZI/CHL = 71.8%; TXC = 41.9% (Figure 5C).
The CV-CARE patients represent a population with significant risk factor burden, with a high number of concomitant diseases and medications. The presence of T2DM, HCh, and HTN significantly increases the risk of cardiovascular disease and mortality [37]. Indeed, the percentage of patients with established vascular disorders was high in the T2DM (53.9%) and HCh (54.7%) patients. However, the percentage of cardiac disorders reported was low at 5.7% (T2DM), 9.7% (HCh) and 4.7% (HTN). This could be explained by the fact that the CV-CARE registry was mainly a primary care registry and patient selection may have favored patients at the primary care stage rather than secondary prevention stage.
Patients reporting T2DM had a disease duration of approximately 5 years, with an A1C at 7.5%. Most patients reported metformin IR use as initial treatment, before switching to MetER. Most patients switched to MetER due to the inability to tolerate metformin IR. The GI side effects of metformin IR have been previously reported in up to 25% of patients [7]. The most common total daily dose was 1000 mg, which is the recommended starting dose. Maximum daily dose is 2000 mg, which 27% of the CV-CARE population reported.
The Canadian screening guidelines for hypercholesterolemia suggest pharmacological intervention to achieve an LDL-C ≤ 2.0 mmol/L, with additional reductions to < 1.4 mmol/L and even < 1 mmol/L being associated with further cardiovascular risk reduction [17,18]. The CV-CARE population reported, on average, a LDL-C of 3.1 mmol/L, a HDL-C of 1.4 mmol/L and a non-HDL-C at 4 mmol/L. Based on the age, blood pressure and diabetes status of this population, most were not at target and required treatment intensification. The Canadian Cardiovascular Society recommends statin up titration or add-on therapy with ezetimibe and/or bile acid sequestrants [14]. In line with this recommendation, approximately half of the CV-CARE population were given C as an add-on therapy (57.1%), although there was still 41.5% on C as monotherapy (41.5%). Colesevelam monotherapy has been shown to reduce mean serum LDL-C levels by 9-19% [3]. However, when used in combination with an HMG-CoA reductase inhibitor (statin), C induces additive reductions in LDL-C that are 10-16% greater than those achieved by monotherapy with a statin [38]. As such, C provides a useful addition to primary therapy with statins in the treatment of hypercholesterolemia. Despite the evidence of the relationship between lower LDL-C and reduced cardiovascular disease risk [39], many patients received treatment that consisted predominantly of statin monotherapy. A large percentage of the registry population reported statin intolerance or contraindication (49.1%) as the reason for using C, in line with the reported poor tolerability of statins in the literature [40]. A large proportion of the CV-CARE population reported taking C in sachet powder form, supporting the patient preference of a drink (for ease of swallowing) versus tablets [41,42].
At enrollment, patients had an average disease duration of 5.5 years at a mean age of 59.6 years. This is not an unexpected finding given that prevalence of hypertension in Canada has been reported to increase significantly from ages 49-84 years [23]. According to Hypertension Canada 2018 guidelines [43], this cohort would be classified as a moderate to high risk population (due to cardiovascular risk factors). In this category, blood pressure threshold for initiation of antihypertensive therapy, in those without diabetes, is ≥ 140 mmHg/≥ 90 mmHg, with long-acting diuretics preferred over shorter acting diuretics, ACE-I, ARBs, long-acting CCB, beta blockers (not first line for those > 60 years) and single pill combinations being recommended [43]. We can see that in the CV-CARE population, multiple first line medications were being used to manage disease, often in combination, which has been shown to minimize counter-regulatory mechanisms that act to restore blood pressure values to pre-treatment levels [44,45]. Most patients require more than one medication to control their hypertension [46]. These anti-hypertensives were most often prescribed for lack of prior treatment efficacy. The most common dose of AZI was 40 mg and the AZI/CHL recommend starting dose of 40/12.5 mg is also the most common reported in the CV-CARE population. The most common total daily dosing of TXC was 240 mg despite blood pressure recordings being above target. As the anti-hypertensive effect of TXC is dose related, and higher doses of 280 mg, 300 mg and 360 mg were available, there may have been an opportunity for a greater number of patients to achieve target blood pressure if the dosage had been increased [47]. It has been previously reported that physicians can fail to adequately titrate doses of anti-hypertensives [47].
There are several limitations to this study. As this data comes from a registry there are potential biases from treatment selection (health care professional, patient and registry inclusion criteria), reporting bias (health care professional), and participation bias (patients). The registry was observational and as such patients were not randomized between the treatment groups and time between study entry and follow-up visits varies between participants. As this was taking place during routine clinical care, not all parameters were collected for all patients, leading to missing data. The analysis is retrospective and descriptive in nature only. Finally, this registry is Canada-based and specific to BHC CV/MET portfolio, and as such, data should not be extrapolated.
MB (Maxime) and JB conceived the study in collaboration with Drs. Bell, Goldenberg and Cheng. Dr. Cheng was also involved in patient recruitment and follow up. JSS Medical Research was hired to run the study, prepare the data and perform all statistical analyses. EP oversaw individual clinical site monitoring as part of the study's Clinical Trial Associate team. MB and JB interpreted the results. MB reviewed the literature and wrote the manuscript with the assistance of JB and EP. MB, JB, EP, RG, WC, NFA, and AB critically revised the manuscript for scientific quality and content. All authors approved the final version for publication.
This study was sponsored in full by Bausch Health (formerly Valeant Pharmaceuticals LLP), Canada.
RG reports research support from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Janssen, Medtronic, Merck, Novartis, Novo Nordisk, Roche, Sanofi, and Takeda; serving on advisory panels for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, Roche, Sanofi, and Takeda; speaker bureaus for Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, Sanofi, and Servier; consulting for AstraZeneca, Bausch Health, Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk, and Takeda. AB has received research support, consulting fees and/or speaker honorarium from the following commercial organizations: Amgen, Bristol Myers Squibb, Janssen, AstraZeneca, Pfizer, Bayer, Lilly, Boehringer Ingelheim, Canopy Growth, Sanofi, Bausch Health. WC has no conflicts of interest to declare. MB (Melonie Burrows), EP and NFA are employees of Bausch Health Companies. JB and MB (Maxime Barakat) are employees of, and shareholders in, Bausch Health Companies.