The diagnosis of a neck mass can present a challenge. In the adult the most common diagnosis is malignancy, and both primary and metastatic tumors should be considered. Other frequent options are infectious processes. We present the case of an 88-year-old female patient with a submandibular mass with inflammatory signs, unresponsive to antibiotic therapy, with fine needle aspiration biopsy showing an inflammatory lesion. The mass developed over a month with associated anorexia resulting in admission for diagnosis with open biopsy. Following admission, an invasive carcinoma of the right breast was diagnosed, and the Mycobacterial culture of the submandibular mass biopsy was positive for Mycobacterium tuberculosis. Immunosenescence the elderly contributes to vulnerability to cancers but also infections which can present atypically and thus result in delayed diagnosis.
The differential diagnosis of neck masses is extremely challenging, as several factors must be considered. The main diagnostic options in adults is malignancy, either primary, metastatic from tumors of the upper respiratory or digestive tract, or lymphoma [1]. Benign neoplasms such as lipomas, fibromas and hemangiomas [2] can also be found.
Seventy-five percent of the neck masses in patients over 40 years of age are malignant [1], and the risk increases with age [3]. Size of lesion and duration of symptoms are other important predictors of the risk of malignancy [4], as well as chronic sun exposure, smoking, alcohol consumption, poor dentition, environmental exposures and family history [5].
The physical characteristics of the mass are also important for differential diagnosis. Solid, hard, or fixed, with irregular limits are suggestive of solid malignancies; multiple soft and rubbery masses suggest lymphoma; masses with inflammatory signs suggest infectious causes [6].
Inflammatory masses can result from inflammation of lymph nodes (cervical adenitis), which are usually self-limited and resolve spontaneously [2]. Chronic inflammation of submandibular or parotid glands with chronic sialadenitis is also possible [2].
Infectious causes can be viral, bacterial or fungal in nature [2,6]. Viral infections such as Cytomegalovirus (CMV), Epstein-Barrvirus (EBV), measles, Adenovirus, Echovirus, Rhinovirus and Respiratory Syncitial Virus (RSV) [2,6] usually present with multiple lymph nodes with cervical adenitis. Bacterial infections can cause necrosis, with abscess formation, spontaneous drainage and even chronic fistula formation [2]. Other agents should also be considered namely mycobacterial [2,6], cat-scratch disease by Bartonella [6,7], actinomycosis [2,6], Toxoplasma gondii [8].
Other less frequent causes are gout [9], inflammatory pseudotumor [10], Kimura's disease [11], Castleman's disease [12] and sarcoidosis [13].
Diagnosis is usually made by fine needle aspiration cytology, which is a rapid and sensitive method [1,6]. If the diagnosis remains unclear, an open biopsy may be necessary [1,6]. Laboratory tests vary with presentation, but should include inflammatory parameters including leukocyte count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), viral and bacterial serology according to clinical history and examination and tuberculin skin test [6]. Characterization of the mass can be done with ultrasound which differentiates between cystic lesions, salivary gland tumors, reactive or malignant lymph nodes using criteria such as size and vascularization [6]. Computer tomography (CT) scan and magnetic resonance imaging (MRI) further helps in characterization of the masses according to size and morphological abnormalities (central necrosis, fat stranding and heterogeneity) [14]. Positron emission tomography can be used to study neck masses as it detects increased metabolism [6], however it is not recommended as a standalone test, because it does not distinguish inflammatory from neoplastic lesions [6]. If malignancy is suspected, further studies should be carried out, including full body CT scan and endoscopic studies from upper respiratory and digestive tract [1].
We present the case of an 88-year-old frail female livingin a nursing home, who presented to the emergency department due to a 3 to 4-week progressive course of prostration, anorexia and a right submandibular mass with inflammatory signs. She was being studied by the Maxillofacial Surgery Department, who had performed a fine needle aspiration cytology of the mass which revealed inflammatory cells, negative for neoplastic cells. Furthermore she had already been medicated with oral amoxicillin-clavulanic acid (875 mg/125 mg 3id for 7 days) without improvement.
Her past medical history included essential hypertension, sinus bradycardia, peripheral arterial disease, mild cognitive impairment and depression. She was polymedicated with aspirin, olmesartan, pentoxifylline, paroxetine and alprazolam.
On examination she was obese, exhibited a non-tender submandibular mass of 3 × 3 cm, solid but rubberish, with inflammatory signs, without exudate (Figure 1). She remained afebrile with no other relevant clinical signs.
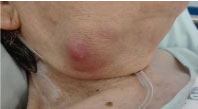 Figure 1: Photo of the right submandibular mass.
View Figure 1
Figure 1: Photo of the right submandibular mass.
View Figure 1
Considering the progression of the disease the patient was admitted for further tests, including an open biopsy of the mass, and was started on empirical treatment with intravenous amoxicillin-clavulanic acid (1000 mg/200 mg for 9 more days) and clindamycin (600 mg 4id for 7 days), also without clinical improvement.
Blood tests showed only a microcytic hypochromic anemia with iron deficiency and negative inflammatory parameters (Table 1).
Table 1: Blood work from the patient. View Table 1
A full-body Computer Tomography (CT) revealed multiple necrotic cervical lymph nodes, the biggest in the left supraclavicular fossae and a nodule in the right mammary gland, irregular with hyperattenuating sign suggestive of vascularization (Figure 2 and Figure 3).
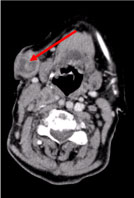 Figure 2: Neck CT scan: Multiple necrotic cervical lymph nodes, the biggest subcutaneous, 27 mm in diameter (arrow).
View Figure 2
Figure 2: Neck CT scan: Multiple necrotic cervical lymph nodes, the biggest subcutaneous, 27 mm in diameter (arrow).
View Figure 2
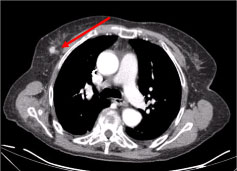 Figure 3: Chest CT scan: 10 mm lesion on the right mammary gland, nodular irregular, with a hyperattenuating sign suggestive of vascularization (arrow).
View Figure 3
Figure 3: Chest CT scan: 10 mm lesion on the right mammary gland, nodular irregular, with a hyperattenuating sign suggestive of vascularization (arrow).
View Figure 3
An open biopsy was performed, which showed soft tissue infiltration by polymorphous nuclear leukocytes and histiocytes and vasculitis. Gram, Grocott, Ziehl-Neelsen and PAS stains were negative; polymerase chain reaction (PCR)-based assay for the detection of Mycobacterium tuberculosis in tissue was also negative.
The patient was discharged with further follow up in the Breast Surgery and Maxillofacial Surgery clinics.
At the Breast Surgery clinic, an ultrasound-guided core-needle biopsy of the breast lesion was performed. Invasive breast carcinoma NOS, grade 1 was diagnosed. The tumor was ER-positive (90%), PR-positive (90%), HER2 2+ by immunohistochemistry, ISH negative, with a proliferation index (Ki67) of 10% (luminal A molecular subtype) (Figure 4). It was staged cT1N0M0. At this time, the patient was started on tamoxifen.
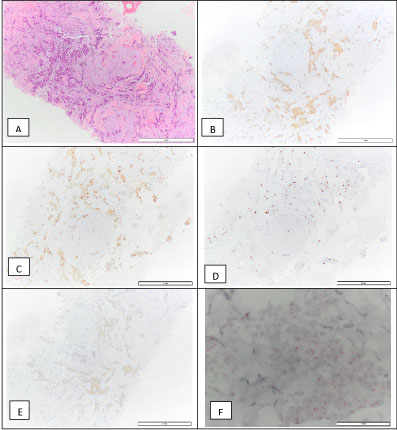 Figure 4: Breast invasive carcinoma biopsy: H&E (A), positive for the estrogen receptor - 90% (B), positive for progesterone receptor - 90% (C), Ki67 - 10% (D), HER2 2+ (E), and a negative D-ISH HER2 amplification (F), which is compatible with a luminal A molecular subtype.
View Figure 4
Figure 4: Breast invasive carcinoma biopsy: H&E (A), positive for the estrogen receptor - 90% (B), positive for progesterone receptor - 90% (C), Ki67 - 10% (D), HER2 2+ (E), and a negative D-ISH HER2 amplification (F), which is compatible with a luminal A molecular subtype.
View Figure 4
Two months later, the mycobacterial culture of the open biopsy was positive for Mycobacterium tuberculosis complex, sensitive to all first line antibiotics. The patient initiated therapy with first-line antituberculostatic agents (300 mg of isoniazid, 600 mg of rifampicin, 1500 mg of pyrazinamide and 1200 mg of ethambutol for 2 months, followed by 4 months of 300 mg of isoniazid and 600 mg of rifampicin) and was referred to the Tuberculosis clinic for follow-up.
The patient died 1 year after the initial diagnosis.
As previously discussed, the diagnosis of a neck mass is affected by several factors. Age and frailty are major factors to consider.
Ageing is associated with a decrease in the immune response, often described as Immunosenescence [15-17]. It affects both the adaptive and innate systems, in differing ways [15,17,18]. The adaptive immune system is often decreased in function, usually by a decrease in T cell activity [15,16]. On the other hand, the innate immune system has been described as being excessive by lack of regulatory power, which may result in apro-inflammatory state, which is also detrimental [15]. Immunosenescence has been associated with an increase in frequency and severity of infections [18], a lower immune surveillance of malignant cells and a decreased efficacy in vaccination [19].
Therefore, immunosenescence contributes to the increased diagnosis of cancer in the elderly, with a median age of 70-years-old at diagnosis [16,17,20]. Other factors play a role, such as the increased time exposed to carcinogenesis or higher susceptibility of older cells to carcinogenesis [17,21], but also the increased paradoxical inflammatory response [17,22].
Infections are more frequent in the elderly, in particular respiratory tract infections [23], with great mortality [24] associated. Tuberculosis is an important public health problem affecting 9.6 million people [25] worldwide and has been increasing in the population above the age of 65 [26]. It has been suggested that tuberculosis in the elderly often results from reactivation of latent tuberculosis, as the cellular immune response wanes [26-30]. However, it can also result from primary infection or reinfection on a previously treated patient [28,29]. Other factors increase the risk of tuberculosis in the elderly, such as residing in a nursing home, which increases the risk 2-3 fold [28-30]. Some comorbidities, like diabetes mellitus [31], chronic obstructive pulmonary disease [32], liver disease, malignancy and cardiovascular diseases [25]; immunosuppressive therapies with corticoids and anti TNF [33]; and malnutrition [25,27-29].
Tuberculosis in the elderly has an atypical clinical presentation [27,34]. The main presentation is still pulmonary in 75% of cases [35], but extrapulmonary sites are more frequent [25,26,36]. Organ specific symptoms are less frequent, and patients exhibit more vague symptoms, such as asthenia and cognitive impairment [25,26,29,37]. Diagnosis is also more difficult as the elderly have frequent chronic abnormalities, such as abnormal liver enzymes, hypoalbuminaemia, hyponatraemia, hypokalaemia, anemia [34]. Radiographically, there are fewer chronic changes [25,38]. The tuberculin skin test is often undetermined [26] due to anergy, and there is a need for a repetition taking advantage of the boost effect [39]. Specimen cultures can be negative [26]. Treatment is also a challenge in the elderly, due to poorer compliance and polypharmacy with increased risk of toxicity, especially hepatotoxicity [25]. Mortality is also significantly higher; by up to 10-fold [40].
This case report illustrates the challenges of diagnosing a neck mass in the elderly. Local inflammatory signs were suggestive of infection, but the systemic symptoms raised the suspicion of malignancy. In hindsight, we may argue that several signs and laboratory tests could suggest tuberculosis, such as inflammatory signs, resistance to antibiotics, anemia and hyponatremia. Simultaneous diagnosis of invasive breast carcinoma was an incidental finding. However, it is reasonable to assume that cancer may have contributed to immunosenescence, facilitating the reactivation of latent tuberculosis.
There was no financial support.
All authors contributed equally.