Background: Immunotherapy has played a pivotal role in the treatment of different malignancies. However, agents such as Nivolumab have the potential to potentiate the immunomodulatory response and can lead to various adverse effects, such as acute interstitial nephritis or colitis.
Case presentation: A male in his 70s with a history of hypertension, ulcerative colitis, diabetes mellitus, elbow melanoma with axillary lymphadenopathy status post excision on nivolumab therapy (completed 12/13 doses), presented after outpatient bloodwork revealed elevated creatinine within the context of one week of diffuse, watery diarrhea. Patient's baseline creatinine one month ago was .82, but on admission it was 6.77 mg/dL. He denies changes in urination, hematuria, or dysuria. On admission, labs were significant for potassium of 5.5 mmol/L, blood urea nitrogen of 92 mg/dL (26 previously), and creatinine 6.77 mg/dL (0.82 one month prior). Urinalysis was significant for 64 white blood cells/high power field (hpf), and sterile pyuria. Colonoscopy showed erythematous mucosa in the rectum and rectosigmoid junction, and pathology showed active colitis. Interleukin 2 receptor was elevated at 5475 pg/mL. Gallium scan showed increased radiopharmaceutical accumulation in both kidneys suggestive of acute interstitial nephritis. He was treated with high dose steroids and his creatinine improved to 1.22 mg/dL on outpatient follow up two months later.
Conclusions: Nivolumab therapy may cause acute interstitial nephritis and colitis, even after 12 tolerated doses. Gallium-67 scintigraphy may help support the diagnosis non-invasively. Patients may have a complete recovery to normal with the use of high dose steroids and supportive treatments.
Immunotherapy, Nivolumab, Melanoma, Colitis, Acute kidney injury, Interstitial nephritis
Immunotherapy has played a pivotal role in the treatment of different malignancies. However, agents such as Nivolumab have the potential to potentiate the immunomodulatory response and can lead to various adverse effects, such as acute interstitial nephritis or colitis.
A male in his 70s with a history of hypertension, ulcerative colitis, diabetes mellitus, elbow melanoma with axillary lymphadenopathy status post excision on nivolumab therapy (completed 12/13 doses), benign prostatic hyperplasia, presented after outpatient bloodwork revealed elevated creatinine within the context of one week of diffuse, watery diarrhea. Patient’s baseline creatinine one month ago was 0.82, but on admission it was 6.77 mg/dL. He states that he has been asymptomatic except for the last week, where he has been having intermittent diarrhea. He denies bloody diarrhea. He denies abdominal pain, nausea, vomiting. He denies changes in urination, hematuria, or dysuria. He has been receiving monthly immunotherapy infusions since he was diagnosed with melanoma 1 year ago. He was supposed to get his last infusion today (13 th dose).
On admission, labs were significant for hemoglobin 10.5 g/dL, sodium 133 mmol/L, potassium of 5.5 mmol/L, blood urea nitrogen of 92 mg/dL (26 one month prior), creatinine 6.77 mg/dL (0.82 one month prior), phosphorous of 5.9 mg/dL. Urinalysis was significant for 64 white blood cells/high power field (hpf), 1 red blood cell/hpf, 5 epithelial cells/hpf, negative bacteria, negative nitrite, and large leukocyte esterase consistent with sterile pyuria. Urine culture was negative for bacterial growth. Colonoscopy showed erythematous mucosa in the rectum, rectosigmoid junction, and sigmoid colon and pathology showed active colitis with hypercellular lamina propria. Interleukin 2 receptor was elevated at 5475 pg/mL. Urine protein/Creatinine ratio elevated to 0.6. Fractional excretion of sodium was 7.7%. Kidney and bladder ultrasound showed bilateral renal parenchymal disease, no hydronephrosis, chronic bladder outlet obstruction. Gallium scan (Figure 1, Figure 2 and Figure 3) showed increased radiopharmaceutical accumulation in both kidneys, more intense than activity in the lumbar spine, suggestive of acute interstitial nephritis.
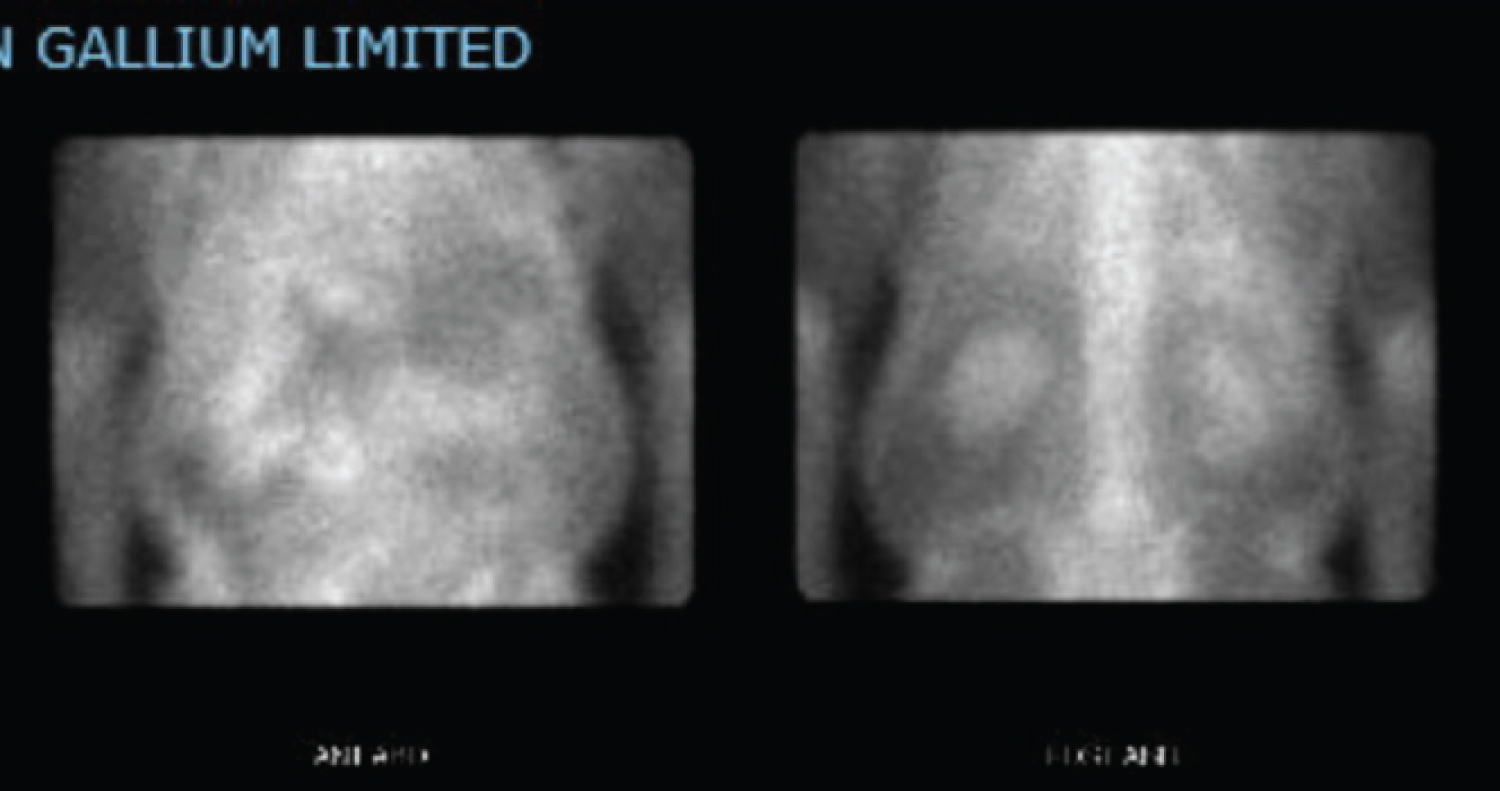 Figure 1: Gallium scan showing renal uptake of radiotracer.
View Figure 1
Figure 1: Gallium scan showing renal uptake of radiotracer.
View Figure 1
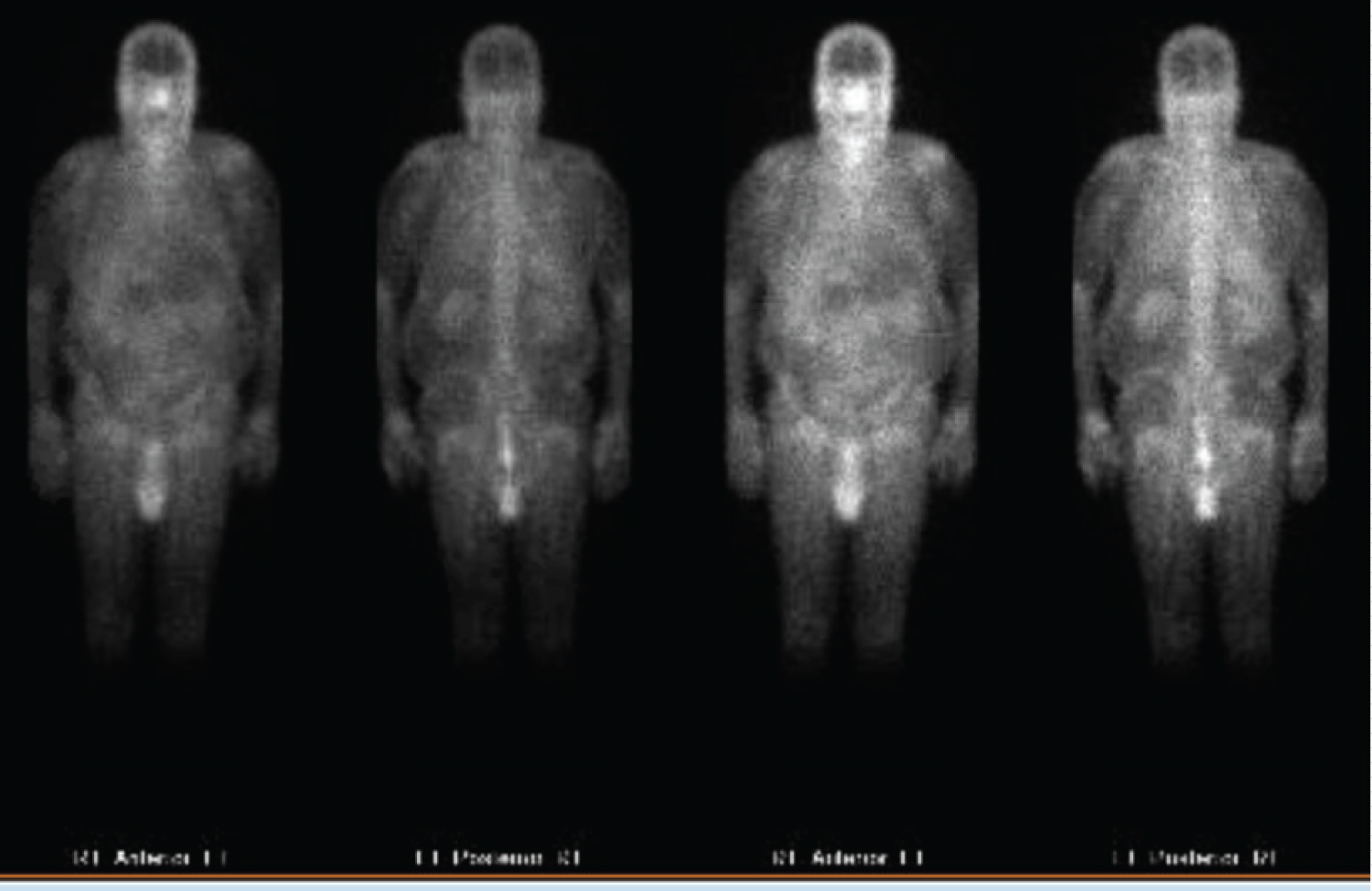 Figure 2: Gallium scan showing renal uptake of radiotracer.
View Figure 2
Figure 2: Gallium scan showing renal uptake of radiotracer.
View Figure 2
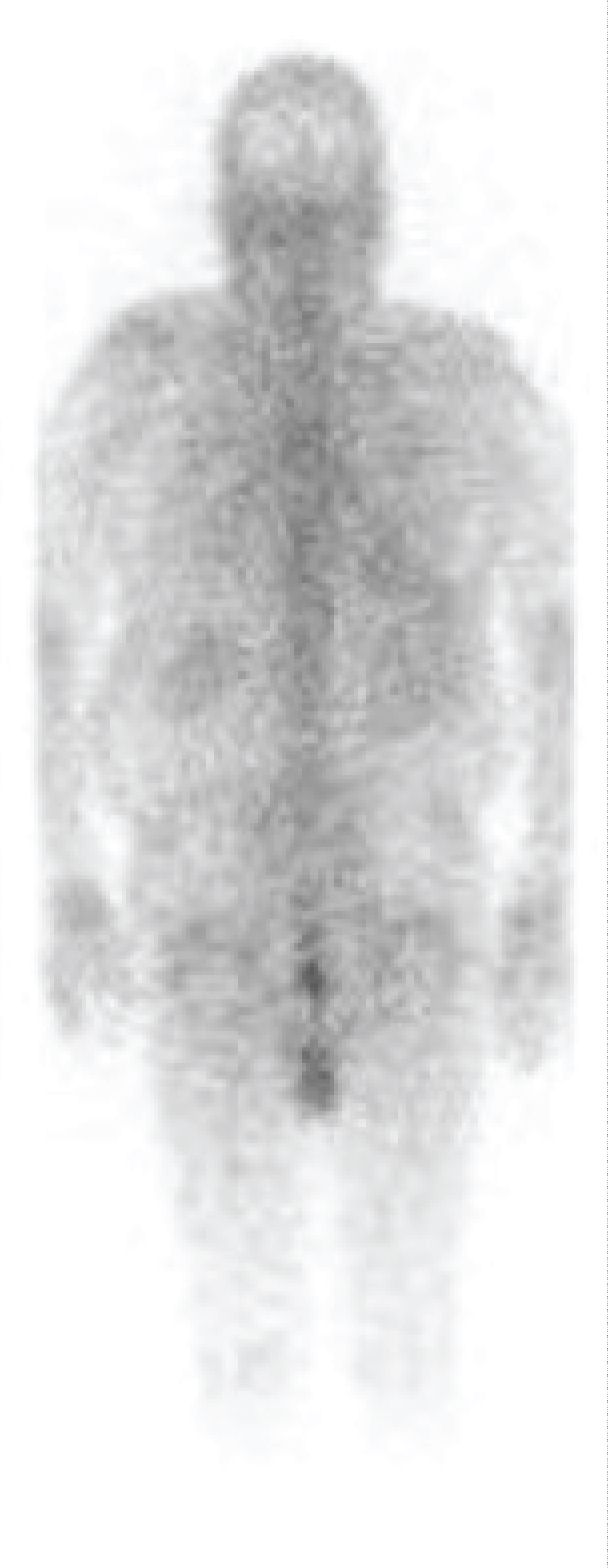 Figure 3: Gallium scan showing renal uptake of radiotracer.
View Figure 3
Figure 3: Gallium scan showing renal uptake of radiotracer.
View Figure 3
Our patient was treated with a bicarbonate infusion, histamine-2 receptor blocker, and Solumedrol 60 mg daily with a significant downtrend in the creatinine to 3.71 mg/dL. Patient was prescribed prednisone 60 milligrams (mg) orally for 4 weeks with nephrology follow up for further management and taper. Patient’s creatinine improved to 1.22 mg/dL on outpatient follow up two months later.
Immune checkpoint inhibitors are immunomodulatory antibodies that help enhance the immune system. These medications have improved the prognosis of individuals with different malignancies, including melanoma. Nivolumab is an antibody against programmed cell death receptor 1 (PD-1). Although it can be useful in treating malignancy through immunologic enhancement, it can also lead to a variety of adverse effects [1].
Immune related adverse events have various timing of presentation anywhere from 21 to 245 days after initiation of therapy [2], so previously tolerated dose of the medication does not predict future responses.
Acute interstitial nephritis is one such complication.
Classic laboratory findings to suggest the diagnosis include urinalysis with sterile pyuria. Other findings include elevated blood urea nitrogen and creatinine, hyperkalemia, hypokalemia, metabolic acidosis, elevated fractional excretion of sodium, eosinophilia, and elevated serum IgE levels [3].
Renal biopsy is the gold standard for diagnosis. However, patients in whom a biopsy is contraindicated (high risk for bleeding, those with a solitary kidney, end stage renal disease, or sepsis), may receive Gallium-67 scintigraphy to help support the diagnosis [4].
Treatment involves withholding the offending medications, hydration, fluid and electrolyte management, and use of steroids such as prednisone 1 milligram per kilogram for one month with a gradual taper. In those who do not respond to steroids, there are case reports that demonstrate benefit with the use of mycophenolate mofetil [5].
A variety of outcomes have been reported. In a case series of 16 patients with acute kidney injury secondary to immunotherapy who were treated with steroids, none of the 5 acute interstitial nephritis cases had complete renal function recovery and 2 of them ended up on permanent hemodialysis [2].
Therefore, prompt diagnosis and management is essential in patients who present with kidney injury from immunotherapy.
Nivolumab therapy may cause acute interstitial nephritis and colitis, even after 12 tolerated doses. Gallium-67 scintigraphy may help support the diagnosis non-invasively. Patients may have a complete recovery to normal with the use of high dose steroids and supportive treatments.
The following case report did not require ethics approval. Consent to participate was obtained from the patient.
Informed consent was obtained from the patient included in this report, including permission for publication of data and clinical images.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
The authors declare that they have no competing interests.
There were no sources of financial support for this case report from government or industry.
JJ was responsible for the writing of the report, data acquisition, and analyzation of data included in this report. All authors read and approved the manuscript prior to submission.
Not applicable.
Authors names and institutions included in this report.