Background: While renal dysfunction has been reported in chronic hepatitis C patients treated with Sofosbuvir-based direct-acting antivirals in high income countries, the impact of this treatment on renal function has not been studied in patients from Sub-Saharan countries such as Cameroon.
Material and method: We conducted a prospective study over 6 months in a single heath care facility in Cameroon. All consenting patients ≥ 18 years treated with Sofosbuvir regimen for 12 weeks were included. Serum creatinine was measured at baseline and during treatment (4th, 8th and 12th week). The Chronic Kidney Disease Epidemiology collaboration equation was used to calculate the estimate glomerular filtration rate (eGFR) and renal dysfunction was defined as a decrease of ≥ 25% eGFR compared to baseline.
Result: A total of 27 patients including. 15 female were studied. The mean age of the patients was 57.9 ± 9.3 years. Overall, we observed a significant on-treatment decline in eGFR (mean eGFR variation: -4.3 ml/min/1.73 m2/month; 95% CI -6.2 to -2.4; p < 0.001) with the lowest eGFR at the 8th week of treatment. Renal dysfunction was noted in 44.5% of participants with a significant on-treatment decline in eGFR (mean eGFR variation -6.9 ml/min/1.73 m2/month; 95% CI -9.5 to -4.2, p < 0.001). There was no difference in age, gender, comorbidities and medication between patients with and without renal dysfunction.
Conclusion: Renal dysfunction is frequent in Cameroonian patients treated with Sofosbuvir, affecting more than 2 patients out of 5 during treatment. Long term renal tolerance and risk factors of renal dysfunction due to Sofosbuvir should be explored in Cameroonian as well as other Sub-Saharan African chronic hepatitis C patients.
Chronic hepatitis C, Sofosbuvir, Estimated glomerular filtration rate, Renal dysfunction, Cameroon
The liver disease caused by the Hepatitis C virus (HCV) represent a major public health concern with an estimated prevalence of 71 million worldwide and over 390,000 deaths annually [1]. Beyond the risk of developing liver complications, mainly cirrhosis and liver cancer, patients with chronic hepatitis C have an increased risk of morbidity and-mortality due to non-liver diseases such as auto-immune diseases, lympho-proliferative disorders (including mixed cryoglobulinemia vasculitis and Frank lymphoma), as well as cardiovascular and renal complications [2]. In the kidney, HCV can induce glomerular damage such as cryoglobulinemic glomerulonephritis (GN), mesangiocapillary GN or membranous nephropathy. The hepato-renal syndrome can also be found as a complication of cirrhosis [2]. Renal complications have also been reported with agents used to treat HCV. Interferon has been associated with minimal change disease, focal and segmental glomerulosclerosis, membranous nephropathy, tubulo-interstitial nephritis, systemic lupus erythematosus-like syndrome and thrombotic microangiopathy [3-5]. Kidney injury has not been attributed to direct-acting antivirals (DAAs) except for Sofosbuvir.
Sofosbuvir is currently the mainstay HCV infection treatment worldwide. It is a nucleotide analogue inhibitor of the HCV non-Structural 5B polymerase with similar in vitro activity against all HCV genotypes [6]. Unlike other DAAs that are mainly of biliary elimination, GS-331007, the main metabolite of Sofosbuvir, is mostly eliminated through the kidney [7]. Data on the nephrotoxicity of Sofosbuvir are still controversial. Nephrotoxicity of Sofosbuvir was not reported as a safety signal in phase III clinical trials; but most of the patients included in the trials had eGFR > 60 ml/min/1.73 m2 and few comorbidities [8-11]. In patients with chronic kidney disease (CKD), Sofosbuvir was found to be safe and effective even in patients with advanced CKD including those on dialysis [12-15]. However, acute Kidney injury (AKI) has been reported to occur in 1-19% of patients treated with Sofosbuvir with a higher incidence among patients with CKD stage 3-4 [7,16-18]. AKI is found to usually occur around 9 weeks after initiation of treatment with Sofosbuvir. It is characterized by histological feature of acute interstitial nephritis and may be reversible following drug discontinuation [16,18]. Baseline CKD, presence of ascites or cirrhosis, concomitant use of nephrotoxic drugs such as non-steroidal anti-inflammatory drugs (NSAID) are considered to be risk factors; and monitoring of kidney function is recommended in all HCV patients treated with Sofosbuvir [7,18,19].
The Prevalence of HCV is reported to be high in Sub-Saharan Africa, especially among central African countries such as Cameroon where the estimated prevalence of HCV is 6.5% [20,21]. DAAs have been recently introduced in Cameroon and Sofosbuvir is the cornerstone of the treatment. Data on the renal safety of Sofosbuvir in Cameroon, as well as in the Sub-Saharan region are not currently available. We therefore sought to describe the evolution of eGFR in patients receiving Sofosbuvir-based DAAs in Cameroon.
We conducted a prospective study over a6 month-period (November 1st, 2019 to April 30th, 2020) at the hepato-gastro-enterology unit of the Yaounde Teaching Hospital, which is one of the main hepato-gastro-enterology services of the Cameroonian capital.
Patients ≥ 18 years with hepatitis C treated with Sofosbuvir-based DAAs for 12 week-period who agreed to participate to the study were included. Patients with known CKD stage 5, those with decompensated cirrhosis and patients who were lost to follow-up before the end of the treatment were excluded. Patients were recruited in outpatient clinics, before the initiation of Sofosbuvir-based DAAs. They were then followed and evaluated at the 4th, 8th and 12th weeks of treatment. Recruitment of patients was completed before the 1st January 2020 and all patients were followed from the beginning to the end of treatment (12 weeks).
Age, gender, comorbidities, medications (NSAID, Metformin, angiotensin blocker, Tenofovir, diuretics), blood pressure (BP) were recorder as well as, HCV viral load, baseline serum transaminase and baseline haemoglobin levels when available. The medical record of the patient was used to complete information obtained during the participant's interview. Blood samples were collected at weeks 0, 4, 8 and 12 of Sofosbuvir-based DAAs treatment for serum creatinine analysis. The daily dose of Sofosbuvir, Velpatasvir and Daclatasvir were respectively 400 mg/day, 200 mg/day and 60 mg/day.
The Jaffe's kinetic method was used to measure serum creatinine. The eGFR was assessed from baseline to end of treatment using the Chronic Kidney Disease-Epidemiology collaboration (CKD-EPI) equation. Renal dysfunction was defined as a reduction of at least 25% of the eGFR when compared to baseline.
Data were analysed with the IBM SPSS (Statistical Package for Social Sciences) version 26.0. Qualitative variables were presented as frequency and percentage while quantitative variables were presented as mean ± standard deviation or median + interquartile range according to their distribution. A generalized linear model was used to determine the variation of eGFR during treatment. Chi tests were used to compare variables among patients with and without renal dysfunction. A p-value of < 0.05 was considered as statistically significant.
Ethical clearance was obtained from the Ethical committee of the Faculty of Medicine and Biomedical Sciences of Yaounde and administrative authorization was obtained from the Yaounde Teaching Hospital. A written informed consent was obtained from each participant.
Sofosbuvir-based DAAs was initiated in 31 adult patients between November and December 2019; 4 patients were lost to follow-up before the end of treatment and a total of 27 participants were included in the study. The mean age was 57.9 ± 9.3 years (range 33-75 years) and 15 participants were female. Comorbidities were noted in 33% (n = 9) of participants with high blood pressure (n = 8, 29.6%) and diabetes (n = 7, 26%) as the most common. Co-infection with Hepatitis B or D virus or HIV was not observed. Angiotensin blockers were the main nephrotoxic drugs used. Use of traditional medicine or non-steroidal anti-inflammatory drug was not reported by patients before and during Sofosbuvir-based DAAs (Table 1). Mean baseline systolic and diastolic BP were respectively 132.3 ± 20.4 mmHg and 82.6 ± 12.4 mmHg with 10 patients above 140/90 mmHg at baseline.
Table 1: Baseline characteristics of participants. View Table 1
The median duration from HCV diagnosis to the initiation of treatment was 6 weeks (25th-75th interquartile (IQR) 3.5-8.5). Genotype was unknown in most patients (n = 15, 55.5%). Among those with available genotype, only genotype 1 (n = 7, 26%) and 4 (n = 5, 18.5%) were found. The median viral load was 1.094.778 (668.760-2.255.336) copy/mm3. All the participants were naïve to HCV treatment. Two Sofosbuvir based-DAA combinations regimen were used to treat the participants (Sofosbuvir + Velpatasvir: n = 22, 81.5% and Sofosbuvir + Daclatasvir: n = 5, 18.5%).
At baseline, 2 patients had an eGFR < 60 ml/min/1.73 m2 amongst which a known CKD stage 3b patient. For the other patients, the mean baseline eGFR was 86.6 ± 17.3 ml/min/1.73 m2.
Overall, there was a significant on-treatment decline in eGFR (mean eGFR variation: -4.3 ml/min/1.73 m2/month; 95% CI -6.2 to -2.4; p < 0.001). The lowest eGFR was noted at week 8 while a slight increase was observed at the end of the treatment (Figure 1).
Among the 2 patients with baseline eGFR under 60 ml/min/1.73 m2; one patient experienced an improvement in eGFR and the other one (female with a known CKD stage 3b) presented a decrease in eGFR from 30.1 ml/min/1.73 m2 at baseline to 19.2 ml/min/1.73 m2 at week 8 and 22.2 ml/min/1.73 m2 at the end of the treatment (Figure 1).
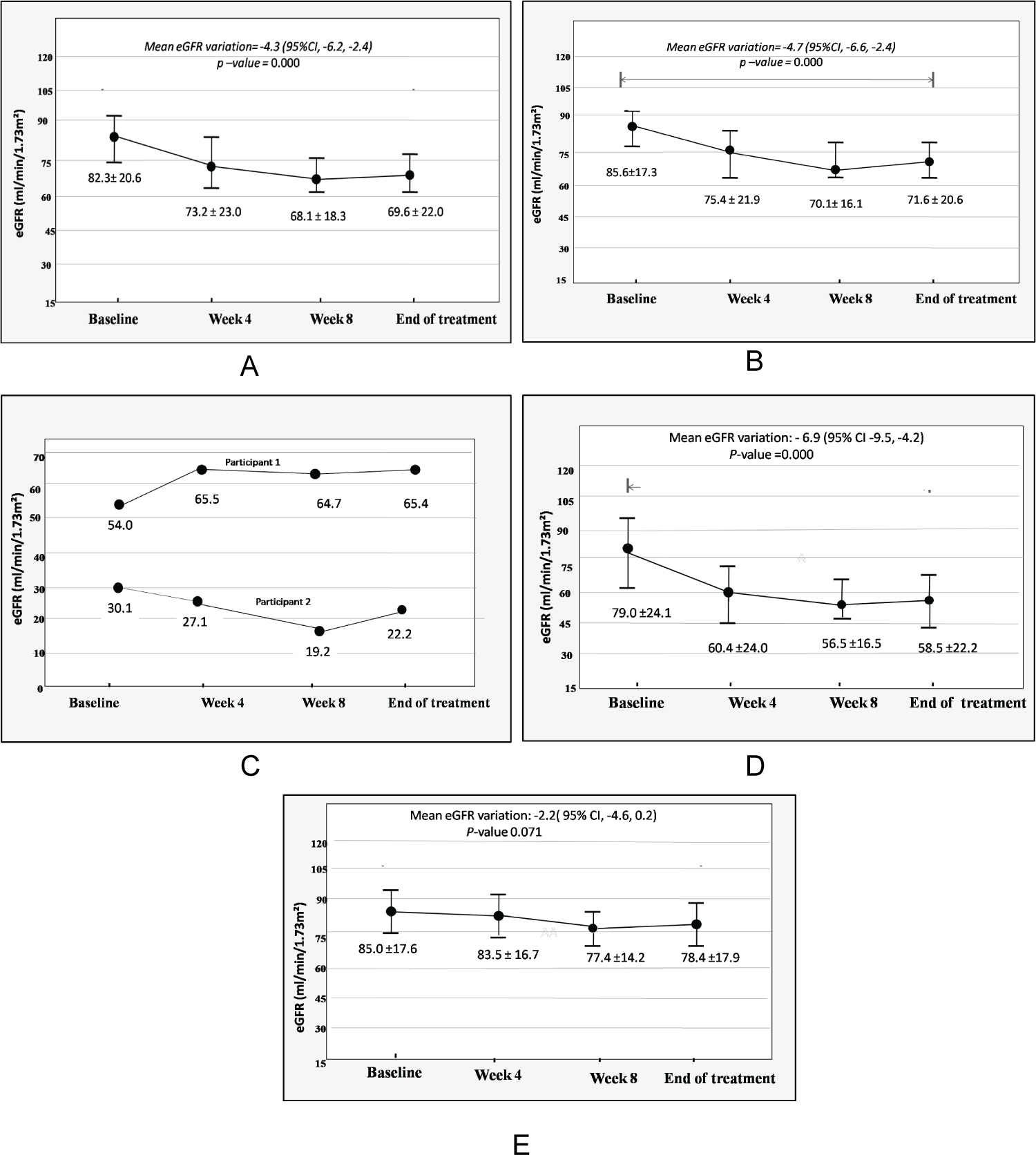 Figure 1: Evolution of eGFR. A) Evolution of eGFR of all the participants (N = 27); B) Evolution of eGFR of patient with baseline eGFR ≥ 60 ml/min/1.73 m2; C) Evolution of eGFR of patients with baseline eGFR < 60 ml/min/1.73 m2; D) Evolution of eGFR in patients with renal dysfunction; E) Evolution of eGFR in patients without renal dysfunction.
View Figure 1
Figure 1: Evolution of eGFR. A) Evolution of eGFR of all the participants (N = 27); B) Evolution of eGFR of patient with baseline eGFR ≥ 60 ml/min/1.73 m2; C) Evolution of eGFR of patients with baseline eGFR < 60 ml/min/1.73 m2; D) Evolution of eGFR in patients with renal dysfunction; E) Evolution of eGFR in patients without renal dysfunction.
View Figure 1
Renal dysfunction was observed in 12 (44.5%) participants with a mean eGFR decline of 6.9 ml/min/1.73 m2/month (95% CI -9.5 to-4.2, p < 0.001) while variation of eGFR was not significant in patients without renal dysfunction (mean eGFR variation: -2.2 ml/min/1.73 m2/month; 95% CI -4.6 to 0.2; p = 0.071) - Figure 1. Age, gender, comorbidities and medications were similar among patients with and without renal dysfunction (Table 2).
Table 2: Comparison of some variables according to on-treatment renal dysfunction. View Table 2
This study sought to evaluate the evolution of eGFR in HCV patients treated with Sofosbuvir-based DAAs in a health care facility from Cameroon. Overall, we observed a significant on-treatment decline in eGFR with a mean eGFR variation of -4.3 ml/min/1.73 m2/month (95% CI -6.2 to -2.4; p < 0.001). Renal dysfunction was noted in 44.5% (n = 12) of participants with a mean eGFR variation of -6.9 ml/min/1.73 m2/month (95% CI -9.5 to -4.2, p < 0.001).
A significant on-treatment decline in eGFR has also been reported by others. In Taiwan, Chen-Hua, et al. noted that patients receiving Sofosbuvir-based DAAs experienced a significant on-treatment decline in eGFR compared to patients receiving Sofosbuvir-free DAAs [22]. Tsai, et al. in Asian chronic HCV cohort patients, found a significant decrease in eGFR from baseline to end of treatment in patients treated with DAAs including Sofosbuvir. However, while an improvement of eGFR was noted with other DAAs, eGFR remained unchanged from end of treatment to 12 weeks after treatment in patients receiving Sofosbuvir [23]. In Portugal, Soeiro, et al. also found a decrease in eGFR in HIV/HCV patients treated with Sofosbuvir + Ledipasvir including those not receiving Tenofovir [24]. Similarly, in France, Mallet, et al. noted a significant on-treatment decline of eGFR in patients receiving Sofosbuvir-based DAAs and Sofosbuvir was a risk factor of eGFR decline in HCV patients treated with DAAs [25].
Two of our patients had baseline eGFR < 60 ml/min/1.73 m2. One patient experienced worsening of renal function with the lowest eGFR at week 8th (30.1 ml/min/1.73 m2 at baseline to 19.2 ml/min/1.73 m2 at week 8 to 22.2 ml/min/1.73 m2 at week 12th); while the other patient had an improvement of his kidney function during treatment (54 to 65.4 ml/min/1.73 m2). As his eGFR increased during the first 4 weeks of treatment and then remained stable, it is possible that this patient experienced an AKI before initiation of the treatment.
AKI may occur in 1-19% of patients treated with Sofosbuvir-based DAAs depending on AKI definition and patient selection with a higher frequency among CKD patients [7]. AKI is usually defined as an increase in serum creatinine by ≥ 0.3 mg/dl within 48 hours or an increase in serum creatinine to 1.5 times baseline within 7 days or decrease in eGFR by ≥ 25% within 7 days [26]. However, most studies in patients receiving DAAs defined AKI as an increase of ≥ 0.3 mg/dl from baseline during or after treatment. Thus, the use of the term 'AKI' can be confusing and other terms such as renal dysfunction may be more appropriate. In our study, 44.5% (n = 12) of participants experienced renal dysfunction. All of them also had an increase of at least 0.3 mg/l in serum creatinine levels when compared to baseline during Sofosbuvir-based DAAs treatment. This percentage of treatment related renal dysfunction is high when compared to previous reports as CKD, the main risk factor of renal dysfunction, was uncommon in our cohort (93% of participants had eGFR ≥ 60 ml/min/1.73 m2). In the USA, Brown, et al. reported that 19% of HCV patients receiving Sofosbuvir + Ledipasvir experienced renal dysfunction during treatment and only 53% of patients with renal dysfunction had improvement in serum creatinine to less than 0.2 mg/dl above their baseline within 12 weeks after the end of the treatment [17]. In their North American study, Maan, et al., found renal dysfunction in 11% of participants with 88% of renal recovery at the end of the study [18]. Saxena, et al. found that 2% of patients receiving Sofosbuvir-based DAAs experienced worsening of renal function with 1% of patients with baseline eGFR > 45 ml/min/1.73 m2 compared to 15% of patients with baseline eGFR ≤ 45 ml/min/1.73 m2 [16]. In CKD stage 3 patients, Shin, et al., reported renal dysfunction in 14.3% of HCV genotype 1 patients treated with Sofosbuvir-based DAAs [27]. Comorbidities, especially diabetes, concomitant use of nephrotoxic drugs (angiotensin blockers, NSAID), ascites, decompensated cirrhosis and baseline renal impairment are usual risk factors of renal dysfunction in literature reviews [7,18,19]. Those factors were infrequent in our cohort, suggesting that other factors such as African ethnicity or genetic factors may be involved in nephrotoxicity of Sofosbuvir in our population.
This study has some limitations. Firstly, we reported a single-center experience and our sample size is small. Therefore, our results may not reflect the true picture of eGFR evolution in patients treated with Sofosbuvir based-DAA in Cameroon. The small size of our sample also limited the ability to conduct a multivariable analysis to identify risk factors of renal dysfunction. Secondly, we did not evaluate the evolution of the renal function after the end of treatment. This would have allowed us to determine if eGFR improved among patients with renal dysfunction. However, to our knowledge, this is the first study that evaluated the evolution of eGFR in HCV patients receiving Sofosbuvir based-DAA in Sub-Saharan Africa. Our result of 44.5% of renal dysfunction during treatment is of interest and further studies should be done to confirm our results, identify factors which could explain this high frequency of renal dysfunction and evaluate the long term renal tolerance of Sofosbuvir in our settings.
The eGFR should be closely monitored in Cameroonian HCV patients receiving Sofosbuvir-based DAAs since we found a significant on-treatment decline of eGFR with the lowest eGFR at the 8th week of treatments. Renal dysfunction is also common and reported in 44.5% of patients. However, long term renal tolerance and risk factors of renal dysfunction due to Sofosbuvir should be explored in Cameroonians as well as Sub-Saharan African chronic hepatitis C patients.
None.
Conceptualization, AAF; methodology, AAF, FMEHD and KMP software: TTOC validation, AAF, FMEHD and KMP; formal analysis, N.A.; investigation, TTOC and KMP resources: AAF, TTOC, FMEHD and KMP data curation, AAF and NNAW; writing - original draft preparation, FMEHD and NNAW writing - review and editing, FMEHD and AAF visualization, KMP and NNAW supervision, AAF; project administration, TTOC and KMP.