The prevalence of twin pregnancies is rising globally due to increased assisted conception and advanced maternal age in pregnancy. Twin pregnancies have 5-9 times higher frequencies of fetal chromosomal and structural abnormalities, often deliver preterm, and have high prevalence of gestational diabetes mellitus (GDM), preeclampsia (PE), and intrauterine growth restriction (IUGR) compared to singletons. Twins have special complications such as twins-to twin transfusion syndrome in mono chorionic (MC) twins. Consequently, more twins are born prematurely, suffer from low birth weight, cerebral palsy, etc., often requiring admission to newborn intensive care units, and develop motor and cognitive disorders for life. These impose a huge burden on individuals, families, and the society.
The Pre-Twin Screen project aim to develop a multi-marker, personalized, prenatal diagnostics model to predict feto-maternal complications in twins. A multi-national network will recruit a statistically powered cohort of 1,200 twins and follow them through pregnancy up to delivery utilizing prior risk, biochemical, endocrine, imagine, immunology and cell free DNA (cfDNA) measures. Initial twin cohorts (Prof. Maymon, Israel), revealed good prediction of Down syndrome, PE, and GDM by prior risk, ultrasound and serum markers. We will test all the above, add cfDNA and evaluate structural and chromosomal abnormalities developed by Prof. Kagan (Tubingen, Germany), imagine standardised by Prof.'s Brown and Miron (McGill, Canada) for MC twins, and endocrine, sonographic, and biophysical markers of Prof. Borrell (Barcelona, Spain), and of Dr. Geipel (Bonn, Germany). Prof. Nicolaides (London, UK) will contribute his large twin cohort data. Prof. Cuckle (Israel) will conduct modelling with our large depository to yield risk stratification algorithms for twins, offering personalized, preventive clinical care of feto-maternal disorders. This would enable a paradigm shift in prenatal personalized care for twin pregnancy.
Twin pregnancy, Mono-chorionic twin, Di-chorionic twins, TTTS, Placenta accrete, Vasa previa, Preeclampsia, IUGR, GDM, Preterm delivery, Biomarkers, NIPT, Ultrasound, Doppler
Twins account for 3-4% of all births and over the last two decades the rate of twin pregnancies has increased steadily worldwide essentially due to a widespread conception by in vitro fertilization and use of ovulation drugs, and the increased age of women when they conceive [1]. Twins are substantially at higher risk than singletons for all common feto-maternal pregnancy complications. For example, in twin pregnancies, the rate of preeclampsia (PE), is about 10%, which is 3-times higher than in singletons [2,3]. While in singleton the average gestational age (GA) at delivery is 39 weeks (wks), mean GA for twin delivery is 36 wks [4,5], which is considered preterm, reflecting a 9-times higher prevalence of spontaneous preterm birth (sPTD) compared to singletons [6,7].
Gestational diabetes mellitus (GDM) is accounting for ~ 22% of twin pregnancies compared to 7-11% in singletons [8]. Twins, compared with singletons, have higher rates of stillbirth, neonatal and infant mortality, low birth weight, chromosomal or structural defects [9], and various developmental disorders [1]. In addition, certain pregnancy complications are specific to mono-chorionic (MC) twins, of which the most common is twin-to-twin transfusion syndrome (TTTS), an anastomosis of blood vessels formed between the twins' pregnancy vascular system due to their close proximity, which is associated with death or severe disabilities of the donor or the recipient twin or both [10-12].
Despite the increased risk for pregnancy complications, twins are excluded from almost all major prenatal clinical studies. As a result -the identification and prevention offeto-maternal complications in twins are lagging behind risk prediction, prevention and management of singletons [13,14].
Our working hypothesis is that utilizing a personalized prenatal approach for a multi-marker risk stratification in twin pregnancy we could decrease mortality and morbidity of both pregnant women and of their twin babies. Given the high prevalence of twin pregnancy conceived through the challenges (cost and health) of assisted reproduction [15], the approach will not only save maternal and new-borns life and reduce complications, but it will also be a huge assistance to cost saving and to efficient use of the limited healthcare resources.
Risk prediction tools will enable to identify patients who may be helped by: 1) The use of low dose aspirin to prevent PE [16], prevention of GDM by life style changes, diet and drugs [17], reduce sPTD with vaginal progesterone or placing a pessary or both [18,19], or save fetallife by laser surgery in cases of TTTS [20].
We offer a multi-disciplinary experienced team to embark on the challenge to offer personalized and predictive feto-maternal evaluation starting early in pregnancy and continuing to delivery to enable prevention and treatment.
This is an international collaboration among world-renowned centres specializing in twin pregnancy care. Our team has led pioneering but small-scale twin perinatology studies that were already published in prestigious journals. These paved the way to embark on the Pre-Twin Screen project for combining all the approaches and generate a big data collection for the deciphering of individual risk for the personalised prenatal diagnosis and management of twin pregnancies at a global level. We hope to generate the "Gold Standard" for twin pregnancy management.
Many aspects contribute to the special needs of twins for prenatal personalized medicine as specified in the background. Twins have a 3-9 times higher prevalence of major feto-maternal complications (PTD, PE, IUGR, GDM), frequently ends with low birth weight, cerebral palsy, long stay in NICU, and the lifelong complications that generate heavy economic and emotional burden for individuals, families and the society [1-15]. Twin mothers are often at an increased risk for cardiovascular diseases, diabetes and obesity that shorten their life expectancy by at least a decade [1]. MC twins often develop TTTS leading to the death of the donor, recipient or both twins [10-12]. Our preliminary studies generated different marker profiles of twins compared to singletons and have demonstrated a different biomarker temporal diversity compared to singleton [21-23]. Together we will generate a rich profile of twin specific measures for personalized diagnostics that will serve for 1) Implementing better clinical care, and 2) Future twin research.
The world of prenatal risk stratification and prevention of major obstetrics syndromes in singleton pregnancy has made a huge leap jump in the last decade leading to successful prediction of chromosomal aberrations, PE, GDM, sPTD, and for a significance prevention of PE, sPTD, and of fetal loss for TTTS or maternal death from vasa- previa or placenta accrete. However, the introduction of such innovation for twin pregnancy is legging a long way behind.
We develop a multi-disciplinary approach for personal prenatal risk stratification, and prevention of feto-maternal complications in twin pregnancies, based on an integrated effort by a multi-national medical centres network. We combined local ongoing cohorts to gain a powered, multi-national cohort to enable a paradigm shift in twin pregnancy management. Unlike international studies of postnatal and adult twin cohorts [24], our focus is prenatal.
Our combined efforts are derived of current progress and achievement. Prof. Maymon and partners pioneered the development of algorithms derived of combined sonographic and serum markers, patient demographics, pregnancy and medical history to enable the prediction of chromosomal abnormalities [21], PE [22], and GDM [23] in twin pregnancy. In treating MC twins with TTTS - Prof. Nicolaides is a leading partner in the international group that develop ultrasound measures and intra uterine laser surgery procedures to save either both twins (50%) or at least one (75%), followed by establishing standards and teaching methods to disseminate this knowledge [25,26]. Dr. Giepel improved outcome by offering shunting procedures to reduce secondary complications [27]. In hospital management of vasa-previa, the group of Prof. Maymon is leading the convoy in the use of steroids to reduce prematurity and maternal complications, facilitating fetal tissue maturation and time delivery, preventing emergency delivery by caesarean (C) section [28,29] or maternal death in cases of placenta accrete [29,30]. Prof. Kagan conducted studies to identify twin related structural and chromosomal complications [10] and described new methods that have been adopted by many in the world. He and his team have pointed out to the impact of chorionicity and maternal serum markers on Down Syndrome screening in twin pregnancies in the 1st trimester and identified the discordance in nuchal translucency thickness in the prediction of severe TTTS [31]. Prof. Burrell in Spain also identified many genetic complications in twin pregnancies. He has also conducted large studies to investigate measures of fetal growth in twins [32] to allow close surveillance and time-delivery in cases of IUGR. Prof.'s Brown &Mironin Canada led many sonographic studies showing the impact of IVF on increasing complications in twins, and emphasized the endocrine impact in these cases [33,34].
Taken together, the team adopt all procedures developed by each to create a comprehensive innovative approach with the aim to yield an unprecedented multiple dimensions and systematic way for identification of feto-maternal pregnancy complications in twin, thus offering risk stratification and prediction in twin to enable prevention and treatment, and generate a "gold standard" for broad implementation.
Our working hypothesis is that utilizing a personalized prenatal approach for a multi-marker risk stratification in twin pregnancy, we could decrease mortality and morbidity of both pregnant women and of their twin babies. Given the high prevalence of twin pregnancy conceived through the challenges (cost and health) of assisted reproduction [1,15] the approach will not only save maternal and new born life and reduce complications, but it will also be a huge assistance to cost saving and to efficient use of the limited healthcare resources.
Risk prediction tools will enable: 1) PE prevention by low dose aspirin [16], prohibit GDM by life style changes, diet and drugs [17], reduce sPTD with vaginal progesterone or placing a pessary or both [18,19], or save fetal life by laser surgery in cases of TTTS [20]. We leverage on having a multi-disciplinary experienced team as outlined above.
1. Build a large multi-centre computerized registry of twin pregnancy with a longitudinal data set of the entire pregnancy to determine the base line for unaffected pregnancies compared to feto-maternal complications.
2. Conduct a longitudinal assessment of imaging (chorionicity, placental location, Vasa Previa, TTTS, Doppler blood flow to internal fetal organs including Ductus Venousus, testing of biochemical markers (PAPP-A, PlGF, PP13, Inhibin A, HCG), perform biophysical measures (mean arterial blood pressure (MAP), UTPI), and evaluate immunological markers of white blood cells (mainly natural killer cell (NK cells). Accordingly, using the prospective cohort of all partners we will determine the differences in markers levels for each of the biophysical, biochemical, molecular, and immunological markers that will be standardized (adjusted to MoM) to compare normal outcomes to different pregnancy complications.
3. Assess the major structural and chromosomal defects by anomaly scan, NIPT, NT, chromosomal microarrays, and analysis of carriers of single gene mutations and their progeny.
4. Decipher the contribution of endocrinology and imagine markers regulating fetal growth including blood flow to essential organs, and endocrine markers (such as adiponectin, leptin, PYY, TNF alpha, IL6, etc.), strengthen them with metabolic measures (sugars, LDL, HDL, triglyceride, lipoprotein, and haemoglobin A1C) standardly tested in pregnancy, and combine the entire repertoire to improve accuracy of personalized predicted complications.
5. Develop a differential diagnosis and prediction model(s) from all the above to yield a powerful, multi-disciplinary and personally guided analytical tools for prenatal risk prediction of feto-maternal complications including the evaluation of impact of gender, which appears to affect the severity of the complications [34].
6. Generate a cost-effective economic model for the prediction of pregnancy complications in twin pregnancies.
7. Create a comprehensive mathematical and epidemiological model for a personalized prenatal diagnosis for guiding RCTs for the prevention of twin pregnancy complications with drugs, surveillance, and in utero laser surgery, thus saving fetal and maternal life.
We aim to offer prediction of PE, low birth-weight, sPTD, - GDM and structural and chromosomal abnormalities for future prevention including the reduction of cerebral palsy. The anticipated twin registry and sample bank, and the multi-marker -based analysis and prediction will integrate patients of different origin, thus enabling model refining for many countries and nations. If successful, our study will turn into a paradigm shift of clinical services for twin pregnancies. Thus, when the results will become available, we will proactively cooperate with patients organizations such as the European Foundation for the Care of Newborn Infants (EFCNI) (http://www.efcni.org/ or the Twins and multiple birth association (https://www.tamba.org.uk/) and cooperative act as the advocacy group to turn our project achievement to clinical practice. In addition, evaluating twin pregnancy our way will path the way for multi-center, randomized drug control (RCTs) studies for prevent in pregnancy complications in twins.
We are approaching the individual local ethics committee to obtain the IRB approvalsand upgrade marker measurements, assure training and proper testing. It involves standardized history collection, and verified testing for biochemical, biophysical, imagine, endocrine and immunological measures including cell free DNA (cfDNA). Our pilot will involve algorithms build up on part of the population (approximately 300 twin pregnancies). This will be subsequently validated for the entire cohort. We aim to follow, when appropriate the competing risk model adjusted to twins to enable risk stratification. There is also a plan to generate cost benefit evaluation. At the project ends we aim to turn our achievement into the standards of clinical care for twin pregnancy and introduce differential management for twin pregnancy complications. This will be based on using: (1) Sonography for TTTS, vasa Previa, placenta Accrete, etc. to enable close surveillance or surgery intervention, or (2) Using risk stratification to refer patients to RCT studies to prevent PE, GDM, or sPTD. The Future Care we anticipate is shown in (Figure 1).
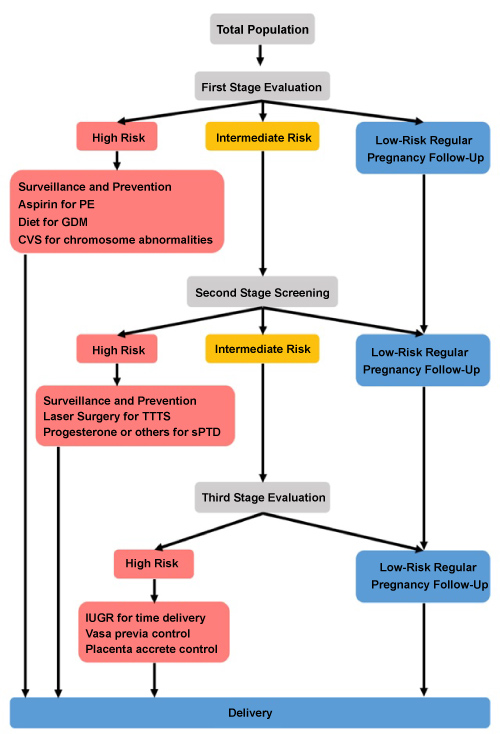 Figure 1: The stage wise risk stratification for Twin pregnancy.
Figure 1: The stage wise risk stratification for Twin pregnancy.
The three-stage model suggests that all twins undergo first stage screening by history, biophysical markers, imaging (chorionicity, nucal translucency, uterine artery Doppler pulsatility Index (UTPI), cervical length, etc.) and biochemical and hormonal markers. Selected proportion of those considered to be at high risk are directed to special surveillances and prevention (PE by aspirin, GDM by diet/drug treatment, sPTD by progesterone). Low risk patients are directed to regular pregnancy follow to delivery. Intermediate risk undergo second stage evaluation.
At the second evaluation by ultrasound and markers, a selected proportion of those identified then to be at high risk are directed to special surveillances and prevention. These includes TTTS separation by laser surgery, preterm delivery prevention with progesterone or other means, close surveillances by ultrasound, monitoring biomarkers for IUGR, and preventing term PE by metformin or methods to recover the normal balance of pro and anti-angiogenic factors, and to diet or insulin to prevent GDM. Those at low risk are directed to regular pregnancy follow up until delivery.
Those with intermediate risk undergo third stage evaluation by ultrasound and markers. The emphasis is on plans for term-delivery for prevention for IUGR, reducing anti-, pro-angiogenic balance to fight term PE, use cervical length measures and steroids to facilitate fetal maturation in cases of vasa previa and placenta accrete, among others. Those at low risk are directed to regular pregnancy follow up until delivery, and those at high risk are directed to close surveillance by ultrasound, blood pressure and urine protein measurements until scheduled for emergency delivery.
View Figure 1
There are no current multi-marker algorithms for the differential diagnostics of feto-maternal complications for twin pregnancies. Leveraging on our former achievements with small cohorts, we will extend the results to reach a global proof using the multi-national, multi-centre project to meet the needs and cost implications of treating the increased revalence of complications in twin pregnancies. Our ideas are linked to the gall of the TAMBA consortium and the Barker's theory that the intrauterine life shape adulthood health, and are the origin for the vicious cycle of non-communicated disorders (NCDs) [35,36] that gained evidence from the 1942-1944 Dutch famine twin cohort reflecting adulthood health complications of the prenatal harsh period [37]. This has shown that improved prenatal care for the vulnerable pregnant women population reduces the prevalence of NCDs in adults [38], and the exponentially increased cost it adds to the global healthcare expenditure [39].
Our working hypothesis estimates possible prenatal prediction of twin pregnancy complications combining patients' demographics, pregnancy and medical history with biochemical, structural and chromosomal abnormalities, immunological, transcriptional, biophysical, and imaging markers that can be incorporated into powerful risk prediction algorithms to direct personal prenatal care, and to reduce feto-maternal morbidity and mortality. Measured individual markers are incorporated as multiples of the median (MoM) for standardization. Mathematical models [40,41] are implemented, and surveillance and prevention models will be offered. As will be further described in the methodology - the larger the risk, the earlier will be the GA at delivery, adding to the increase burden of prematurity [42-50].
The approach was proven successful in singleton pregnancy and here we implement it for twins. We hope to introduce new "gold standards" for personalized feto-maternal prenatal care for twin pregnancy.
This paper is conducted under the support of ERA NET PerMed: Personalised Medicine -JTC2019 pilot, Project number: 061. The Foundation has no influence on the content and the design of this project. All partners declare no conflict of interest.