Graptophyllum pictum is a medicinal plant that helps to cure different forms of disease due to the availability of beneficial phytochemicals such as flavonoids, steroids, alkaloids, saponins and glycosides. The purpose of this research was to evaluate the toxicity effect of G. pictum extract on zebrafish embryos (Danio rerio) at different concentrations. G. pictum was extracted using the ethanol method. A toxicity test was done by exposing the Danio rerio embryo to the G. pictum extraction at different concentrations (0.244-1000 μg/ml) for 24, 48, 72 and 96 hours. The survival rate, hatching rate, heartbeat rate, scoliosis rate and melanin pigmentation were observed. Data were analyzed using SPSS version 27. The value of LC50 was calculated. Result showed that the LC50, value of G. pictum is 7.662 μg/ml. No hatching was observed at higher concentrations (31.25-1000 μg/ml) while the hatch ability of Danio rerio embryos was observed at lower concentrations (0.244-1.953 μg/ml). Scoliosis of zebrafish larvae was not present at all concentrations. The heartbeat of the zebrafish larvae treated with G. pictum extract was within a normal range from 120-180 beats per minute at a lower concentration. Melanin pigmentation was detected at 48, 72 and 96 hours post-fertilization and is normally absent at 24 hours post-fertilization. As a conclusion, G. pictum extract stills exhibit a mild toxicity effect in higher concentrations when it was evaluated on zebrafish embryos.
Graptophyllum pictum, Danio rerio embryo, Heartbeat, Hatching and toxicity effect
Herb-derived medicine is also known as alternative medicine that is commonly practiced in many countries and it plays a key role in the treatment of health and illness. In addition to being a cure for the treatment of an illness that affects humans. In herbal medicine, several useful compounds are made up of natural sources that are advantageous to cure different diseases. Consequently, the worldwide market for herbal medicine has grown dramatically in recent years [1].
Despite the increasing demand from the herbal medicine industry, questions remain about not only their use but also their safety. Herbs are not only medicinally useful ingredients but may also include toxic compounds [2]. After a substantial consumption of heavy metals and phytochemicals, toxic consequences might occur in herbal remedies. Toxicology monitoring is not only essential for allopathic medicine but also herbal medicine. Any of the adverse effects that could be related to the use of herbs may be detected by a toxicity test. The primary aim of the toxicological evaluation of herbal medicinal is to identify adverse reactions, to establish the dose levels at which such an effect exists, to demonstrate the efficacy, and to enhance the usage of herbal medicinal consumption [3].
Zebrafish are often used to investigate the toxic effects and to examine the bioactive compounds of herbal plants on embryonic growth. Zebrafish scientifically known as Danio rerio is a conventional animal model that is extensively used in the biomedical science field for toxicity studies [4]. Based on many advantages, the zebrafish embryo is regarded as a laboratory model of toxicology. These benefits include the rapid embryogenesis process and the output of a large number of eggs at one time, along with less husbandry maintenance. Moreover, the growth of the embryonic process is easy to examine as the embryo is clear and transparent. The eggs are also extremely permeable and tolerant of chemical component absorption [5]. Besides, the zebrafish genome has nearly 70% of homology with humans and 84% of its gene tends to be aligned with human diseases [6]. Some of the main parameters of the toxicity assessment are survival rate, tail formation, scoliosis, heart rate, LC50 determination, melanin pigmentation and hatch in grate of the zebrafish embryo. Zebrafish embryo is an effective assay in the exploration of many diversions of toxicology studies which can give rapid results.
Graptophyllum pictum is an essential medicinal plant belonging to the Acanthaceae family. This plant is usually referred to as a caricature plant and is most likely native to New Guinea, India, Indonesia and widely distributed in some countries. The dried leaves of G. pictum contain some beneficial phytochemicals such as non-toxic alkaloids, pectin, saponins, tannins and flavonoids. These bioactive compounds of G. pictum are responsible for medicinal uses. Traditionally, G. pictum is used as a folk medicine to improve fertility, bruising, wounds, all kinds of swelling and to treat ulcers, abscesses, hemorrhoids, constipation, rheumatism, urinary tract infections, scabies, hepatomegaly, and ear diseases [7]. It may also inhibit the growth of streptococcus mutants. G. pictum has pharmacological properties in antidiabetic, antioxidants, anti-inflammatory and anti-plaque [8]. However, G. pictum does not have enough evidence of research on toxicity effects. The current study is therefore conducted to evaluate the toxic effects of G. pictum ethanol extract on the zebrafish embryo.
The Graptophyllum pictum leaves were collected in UPM, Serdang. Then, the fresh leaves of G. pictum were oven-dried at 40 degrees Celsius (°C). This was followed by powdering the dried sample.
Graptophyllum pictum leaves extraction was carried out using ethanol extraction. The ground form of G. pictum weighing 33g was mixed into 330 ml of 95% ethanol. Then, the mixture was placed in the automatic shaker for 24 hours at 37 °C for 250 rpm. After 24 hours of completed, the extract was filtered three times using Whatman paper. The extract of G. pictum weighing 275.50 ml was placed in the rotary evaporator at 50 °C until the extract becomes waxy. The extract was then placed into an oven at 45 °C for 2 minutes and the dried extract was stored at -20 °C for further use.
The fish tank was cleaned and prepared for the breeding process of zebrafish. The fish tank was filled up with 1000 ml of tap water added with five drops of anti-chlorine. Two male and two female zebrafish were put inside the fish tank. The fish tank is then covered with a box for 17 hours to release hormones. After 17 hours completed, the box was removed and the male and female zebrafish were mating for one to two hours. After the mating process was completed, the zebrafish were taken from the fish tank and the eggs were collected from the fish tank immediately. A mixture was prepared by adding 1000 ml of tap water, a half drop of methylene blue and five drops of anti-chlorine and was transferred into Petridishes. Then, the eggs of zebrafish were placed into the petridishes and were kept at room temperature for 24 hours [9].
A two-fold serial dilution technique was used to acquire a series of G. pictum extract samples with various concentrations. By diluting 0.1g of G. pictum extract with 1000 μl of distillation water, the stock solution was prepared. Twelve successive series of concentration were formed from the 100% solution mixture which were 500 μg/ml, 250 μg/ml, 125 μg/ml, 62.5 μg/ml, 31.25 μg/ml, 15.625 μg/ml, 7.812 μg/ml, 3.906 μg/ml, 1.953 μg/ml, 0.976 μg/ml, 0.488 μg/ml and 0.244 μg/ml. As for the control treatment, distilled water used as negative control and positive control is 100% Dimethyl sulfoxide (DMSO).
Using a pasteur pipette, one embryo was added into each of the 96 well plates at 24 hours post-fertilization (hpf). The treatment was delivered by pipetting 200 μl of a diluted sample into 96-well plates based on various series of concentrations. After 24, 48, 72 and 96 hours of treatment, the embryo was observed under an inverted microscope by focusing on main parameters such as survival rate, melanin pigmentation, hatching rate, heartbeat rate and scoliosis rate.
Data were evaluated using version 27.0 of the SPSS. The data was interpreted as a mean ± SD. All data were interpreted with p < 0.05 suggesting that the result is significant. The LC50 value is the concentration of the sample that causes 50% death in zebrafish. LC50 was obtained with the probit analysis using the mortality value against the test extract concentration.
Based on the result (Figure 1), the survival rate of zebrafish embryo was increased as the concentration of ethanol extract of G. pictum decreased. From 15.625-0.244 μg/ml the survival rate of zebrafish was observed. No development of zebrafish embryos was seen from 62.5-1000 μg/ml. The higher survival rate of zebrafish was seen from 3.906-0.244 μg/ml where as the lowest survival rate was observed from 7.8-15.625 μg/ml. At a concentration of 31.25 μg/ml, 33 percentage of survival rate was observed only at 24 hpf. This indicates that the survival rate of zebrafish decreased as the concentration of G. pictum extract increased. The result of the ethanol extract of G. pictum was shown constant findings at 24, 48, 72 and 96 hpf. As for the positive control, no growth of zebrafish embryo was found after 24, 48, 72 and 96 hpf.
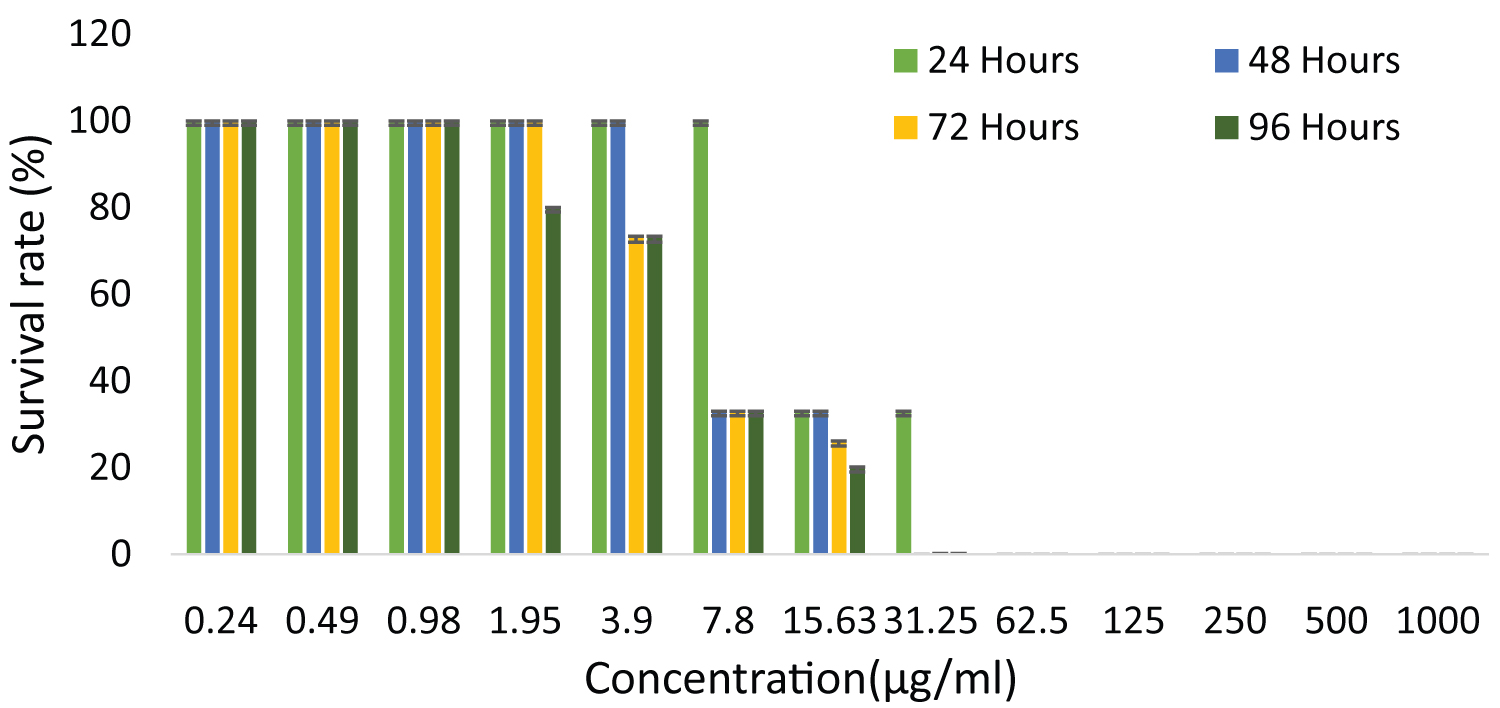 Figure 1: The survival rate of zebrafish embryo examined after exposed to G. pictum at a different series of concentration. Data were expressed as mean ± SD. View Figure 1
Figure 1: The survival rate of zebrafish embryo examined after exposed to G. pictum at a different series of concentration. Data were expressed as mean ± SD. View Figure 1
The hatching rate of zebrafish embryo after exposed to G. pictum extracts with varying concentrations was detected from 48 to 72 hpf. The outcome (Figure 2) revealed that the zebrafish embryo started to hatch from 15.625 to 1000 μg/ml at 48 hpf but the percentage of embryo hatchability was measured at 72 hpf whereas at higher concentrations (31.25-1000 μg/ml), no hatching was observed. However, no hatching was observed in the positive control at 48 and 72 hpf.
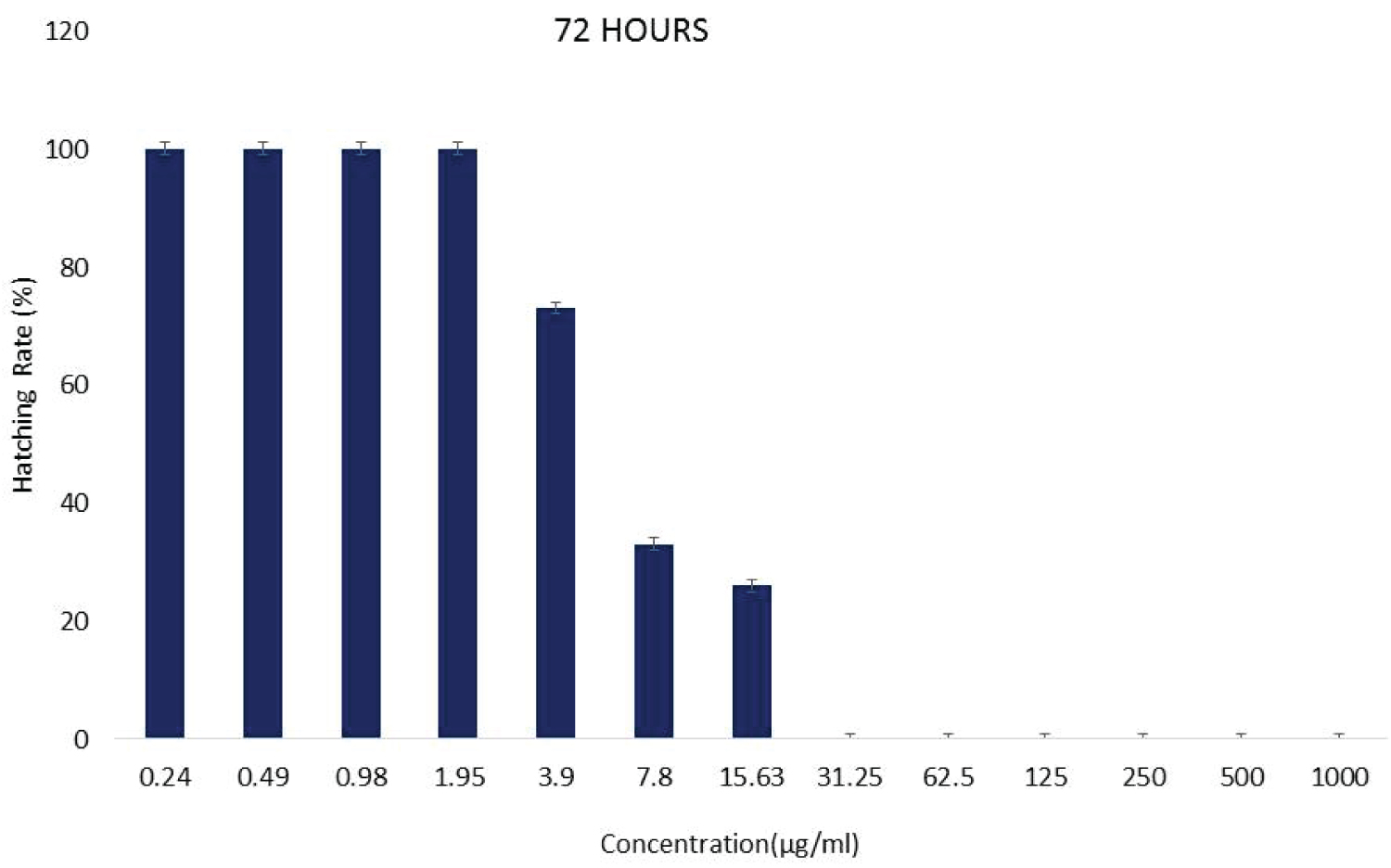 Figure 2: The hatching rate of zebrafish embryo examined after treated with G. pictum extract at a different series of concentration after 72 hpf. Data were expressed as mean ± SD. View Figure 2
Figure 2: The hatching rate of zebrafish embryo examined after treated with G. pictum extract at a different series of concentration after 72 hpf. Data were expressed as mean ± SD. View Figure 2
Based on the result presented in Figure 3, the effect of G. pictum extracts on the heart beat rate of zebrafish embryos was observed at 24, 48, 72 and 96 hpf. The heart beat rate of the zebrafish embryo was normal and was within normal range after exposed to G. pictum extract (0.244-31.25 μg/ml). However, there was no heartbeat rate of zebrafish seen from 62.5 until 1000 μg/ml because it's 100 percent dead. As for the positive control, no heartbeat rate of zebrafish embryo was detected after 24, 48, 72 and 96 hpf.
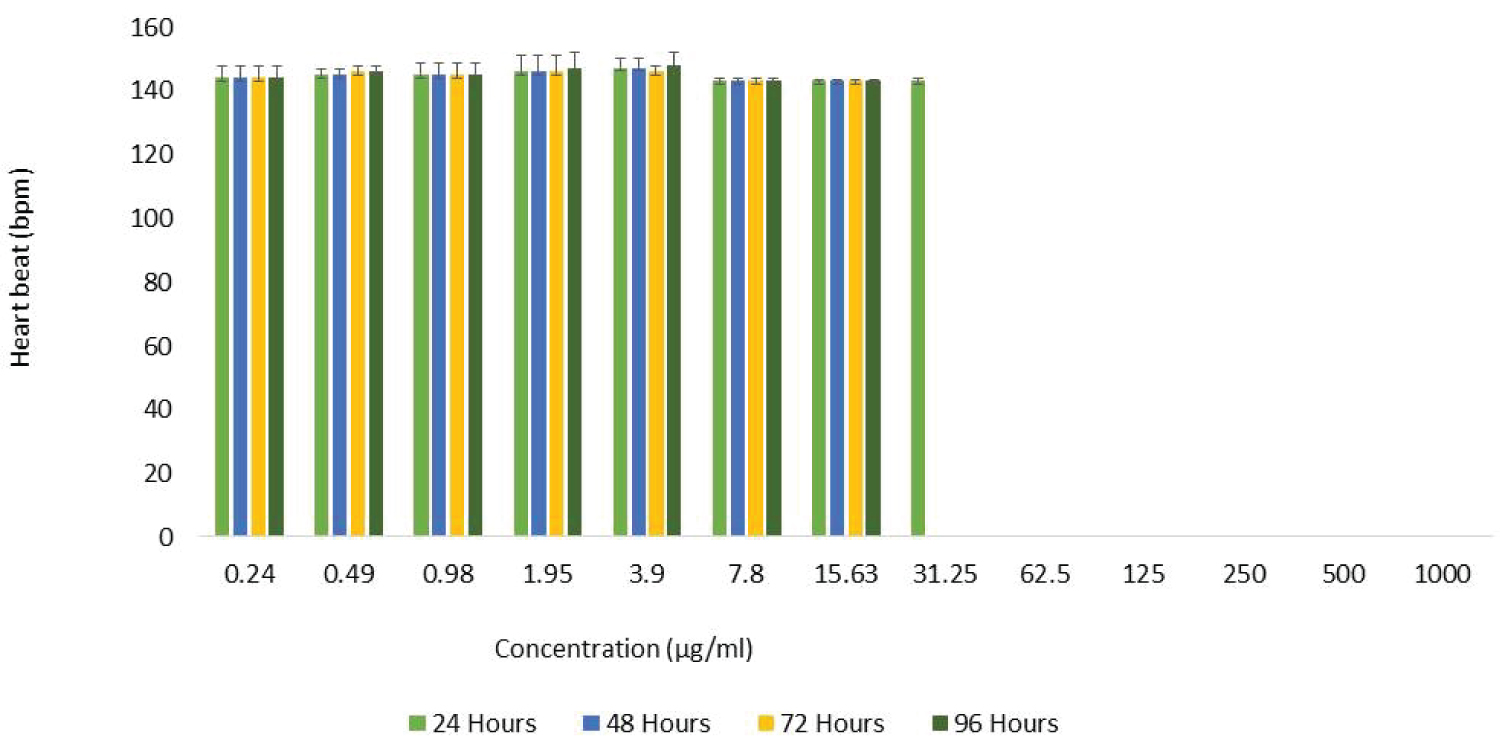 Figure 3: The heartbeat rate of zebrafish embryo examined after treated with G. pictum extract at a different series of concentration. Data were expressed as mean ± SD. View Figure 3
Figure 3: The heartbeat rate of zebrafish embryo examined after treated with G. pictum extract at a different series of concentration. Data were expressed as mean ± SD. View Figure 3
According to the outcome obtained (Figure 4), the LC50 of the G. pictum extract was plotted using the mortality rate against test extract concentration. The LC50 value of ethanol extraction of G. pictum extract was 7.662 μg/ml. The mild LC50 value was observed for the extract of G. pictum. This is because most zebrafish embryos were alive atmost of the tested concentrations (0.244-15.625 μg/ml) after 96 hpf.
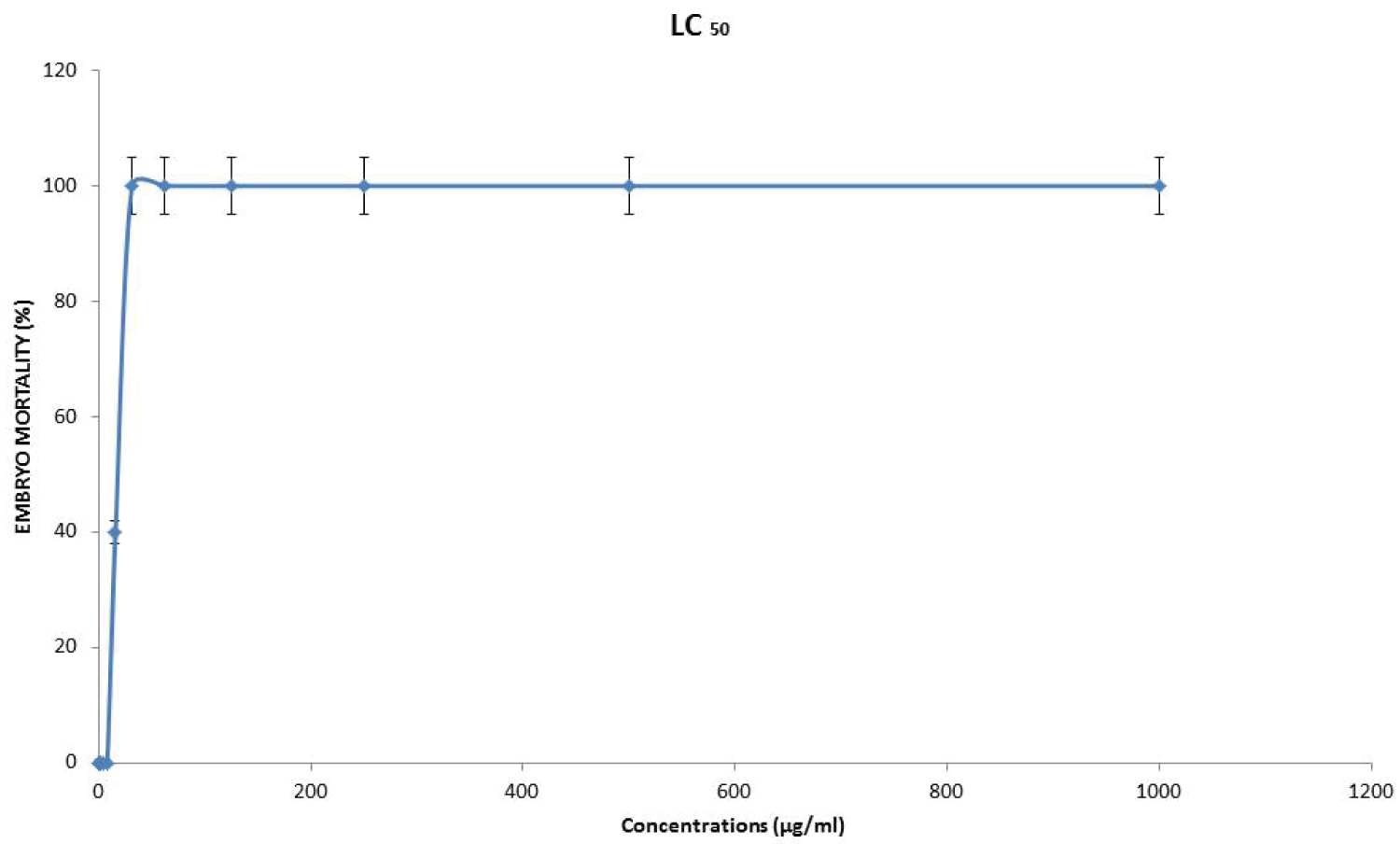 Figure 4: The lethal concentration (LC50) values assessed on the zebrafish embryo model. Results were expressed as mean ± SD. View Figure 4
Figure 4: The lethal concentration (LC50) values assessed on the zebrafish embryo model. Results were expressed as mean ± SD. View Figure 4
The toxic effect of G. pictum extract on scoliosis was determined at 96 hpf. Based on the result obtained (Figure 5), there was no scoliosis observed in all hatched zebrafish embryos (15.625-0.244 μg/ml). As for the negative control, no scoliosis has been seen in the hatched embryos (Table 1).
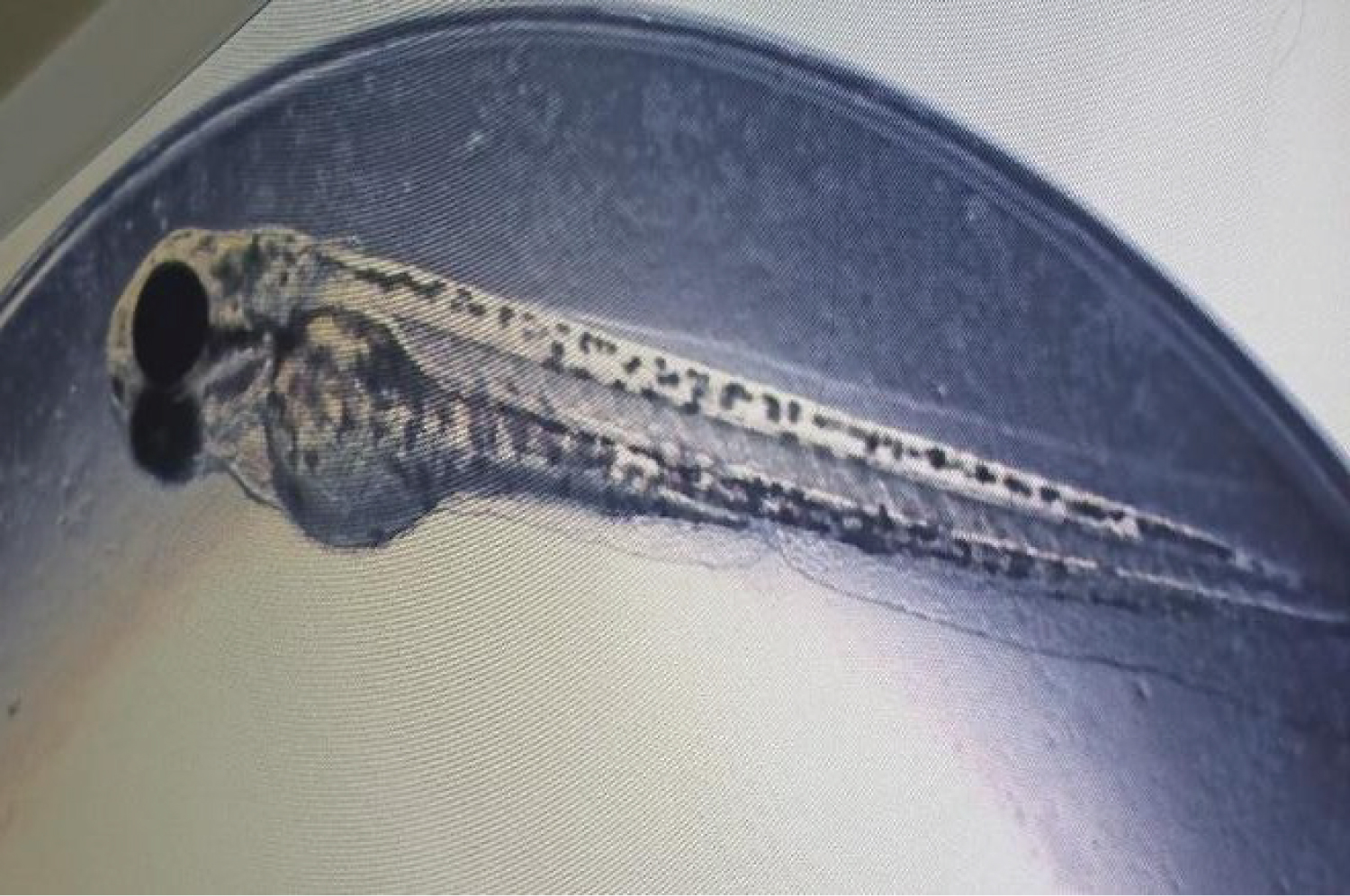 Figure 5: The normal appearance of the hatched embryo without the presence of scoliosis. View Figure 5
Figure 5: The normal appearance of the hatched embryo without the presence of scoliosis. View Figure 5
Table 1: Scoliosis rate of zebrafish embryo after exposed to G. pictum extract. View Table 1
According to the result presented (Figure 6), there was no melanin pigmentation observed on the zebrafish embryo at 24 hpf. The melanin pigmentation was presented on zebrafish embryos at 48, 72 and 96 hpf. No melanin pigmentation was seen at 24 hpf but detected after 48, 72 and 96 hpf for the negative control solution (Table 2).
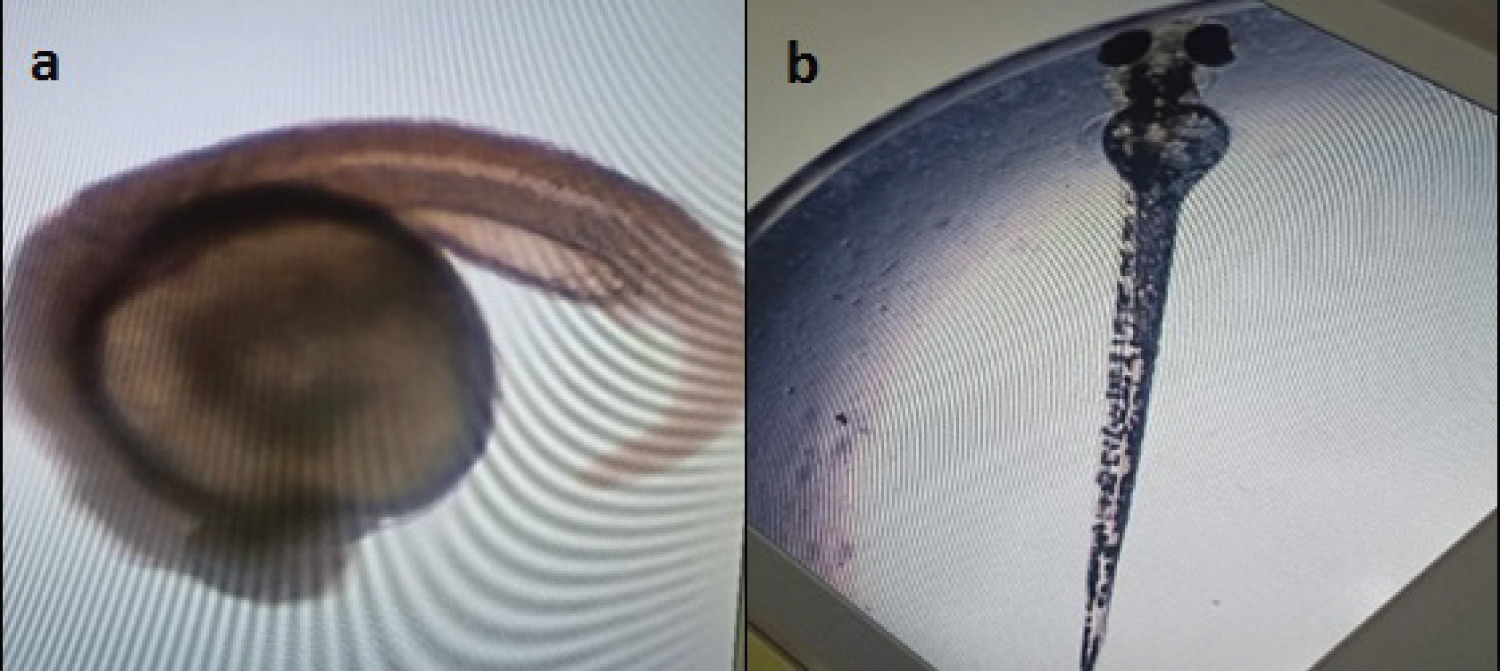 Figure 6: The absence of melanin pigmentation at 24 hpf (a) Compared to the presence of melanin pigmentation at 96 hpf; (b) On zebrafish embryos. View Figure 6
Figure 6: The absence of melanin pigmentation at 24 hpf (a) Compared to the presence of melanin pigmentation at 96 hpf; (b) On zebrafish embryos. View Figure 6
Table 2: Melanin pigmentation rate of zebrafish embryo after exposed to G. pictum extract. View Table 2
As stated earlier, the survival rate refers to the ability of the organism to respond to a given situation in compliance with its optimum state requirement. The pH, temperature, chemistry, and water hardness together with the nitrogenous waste factor are some of the ideal conditions which may determine the survival rate of the zebrafish embryo.
Given the outcome, the survival rate of zebrafish embryos of G. pictum extracts improved as the concentration of test extract declined. 100 percent of the survival rate of zebrafish embryos was found from 0.244 to 0.975 μg/ml. This confirmed that the toxic agents of G. pictum extract do not exhibit toxicity effects on zebrafish embryos at this concentration. From 1.953 to 15.625 μg/ml, the survival rate of zebrafish embryos indicates that toxic compounds of G. pictum extract produce lower toxicity effects on zebrafish embryos. No growth of zebrafish embryo was observed at higher concentrations (62.5-1000 μg/ml). This implied that toxic agents of G. pictum extracts exhibit a higher toxicity effect on zebrafish embryos at higher concentrations. Apart from toxic agents, a higher concentration of bioactive compounds and the heavy metals found in G. pictum leaves also can demonstrate a lower survival rate of zebrafish embryos [10].
Moreover, the negative control showed a survival rate of 100% and this suggests that the negative control was an optimal condition for the survival of zebrafish embryos and signifies that the negative control did not display any kind of toxicity effect. Positive control showed 0 percent development of zebrafish embryos and this revealed that positive control had a greater toxicity effect on zebrafish embryos. A study has shown that DMSO is known to be a low toxicity solvent but the concentration of DMSO should not exceed 0.1% as it may cause toxic effects on animal cells [11].
Hatching is considered to be a crucial stage in zebrafish embryonic development and hence the hatching rate is one of the most significant parameters for determining the toxicity effect of G. pictum extract on zebrafish embryos. A zebrafish embryo is believed to have been hatched until it is fully out of the chorion. Generally, zebrafish embryos begin to hatch at 48 hpf under ideal conditions but the percentage of hatch ability will be evaluated after 72 hpf [12]. The percentage of hatchability will be determined by calculating the number of embryos hatched by the original number of the embryo and multiplying by 100 [13].
Based on the result obtained, from 15.625 to 0.244 μg/ml the zebrafish embryo was successfully hatched at 48 hpf and the percent hatchability was calculated using the formula after 72 hpf. This revealed that the hatching rate shows that the zebrafish embryo did not affect by the toxicity effect of G. pictum extract. There was no hatching observed at higher concentrations (31.25-1000 μg/ml). This implied that a higher concentration of toxic agents, heavy metals and phytochemicals could affect the development of zebrafish embryos. Besides, the observation found that the percentage of hatching rate increased with the concentration of G. pictum reduced which meant that the regular phase of zebrafish embryo hatching was not disrupted. As for positive control, no hatching has been observed which signifies that DMSO is toxic to zebrafish embryos. Conversely, the negative control showed the highest rate of hatching because it is the optimal state for the normal zebrafish embryo hatching process.
Zebrafish is an appropriate cardiovascular research model organism because the heart is the first functioning organ to develop in the zebrafish embryo. The heartbeat of the growing Danio rerio wild-type starts at 36 hpf. The normal range of zebrafish heartbeat is between 120 and 180 bpm which is similar to the human heartbeat. To get the number of heart beats per minute, the number of heartbeats will be counted for 15 seconds and then the number of heartbeats will be quantified by four [14]. The heartbeat of zebrafish can be influenced by some factors such as the compound factor, hereditary mediated temperature adjustment and many others. For instance, room temperature is the ideal temperature for the growth of the zebrafish embryo. The undesirable factors can cause the heartbeat rate either to slow or fast [15]. According to the outcome presented, the heartbeat rate of zebrafish embryos was within the normal range after treated with G. pictum extract (0.244-31.25 μg/ml). This showed that the heartbeat of zebrafish embryos did not affect by chemical factors, the genetic-induced alteration, temperature and many others. For negative control, the heartbeat rate of zebrafish embryos was stable whereas for the positive control, no heartbeat was seen due to 100 percent of embryos dead.
In general, a greater LC50 value of plant extract has a low toxicity level which induces 50 percent of the death of an organism whereas a lower LC50 value contains a high toxicity level which can cause more death of organisms [16]. Based on the outcome, the LC50 value of G. pictum extract was 7.662 μg/ml. This indicates that the 7.662 μg/ml is a medium LC50 value which exhibited mild toxicity effects on zebrafish embryos as well as proved that most embryos were alive at most of the tested concentrations.
Commonly, the irregular curvature of the spine named scoliosis literally indicates that the plant sample is harmful due to the effect of a few surrounding stress factors such as chemical compounds and heavy metals [17]. Based on the current study, there was no scoliosis found in the higher and lower concentrations (15.625-0.244 μg/ml) of all G. pictum extracts which indicates that the plant sample is literally non-toxic. As for the positive control no scoliosis was seen because of 100% dead of embryos and for the negative control, no scoliosis was detected in all hatched embryos.
According to the current research, the melanin pigmentation was present at 48, 72 and 96 hpf whereas it was absent at 24 hpf due to melanocytes initially originating from the neural crest during embryonic development. The eye is essential for organizing the proliferation of neural crest cells in zebrafish. Neural crest cells move anteriorly around the eyes and are not observed on the eye surface until 23 to 24 hpfin wild-type embryos [18]. As for the negative control, melanin pigmentation was absent at 24 hpf and present at 48, 72 and 96 hpf whereas for positive control, melanin pigmentation was not shown because all embryos were dead.
In conclusion, the effect of G. pictum exposure on the zebrafish embryo model through acute toxicity assay assessment was examined in this study. The G. pictum was reported to possess a milder toxic effect. The findings thus suggest that the ethanol extraction of G. pictum is the best test extract as it demonstrated a mild toxicity effect in overall parameters such as survival rate, LC50 value determination, hatching rate, scoliosis, and heartbeat of the zebrafish embryo. Although this plant extract is safe for consumption due to its potential pharmaceuticals, it still induces a mild toxicity effect on higher concentrations as examined in the zebrafish embryo model. A detailed experimental study on phytochemicals analysis should be carried out to determine the specific compounds of G. pictum which possess respective toxic effects.
The authors would like to thank Management and Science University for approval of this study and UniversitI Putra Malaysia for the plant contribution.
There are no conflicts of interest.