Liver is the major detoxifying centre of the body. It removes xenobiotics and their metabolites through metabolism or biliary excretion. Hepatocytes constitute 80% of total liver mass and play a major role in storage, synthesis, metabolism and redistribution of essential molecules. Liver has been known to accumulate > 90% of nanomatrerials translocated from other organs. Bioconcentration of nanoparticles may lead to impairment of structure and function of hepatic cells. Therefore, it is critical to review the information available on NP induced hepatotoxicity, underline the gaps in our knowledge on their toxicity and propose future strategies for nanosafety.
Present review discusses recent researches available on the hepatotoxicity of engineered nanoparticles viz. carbon nanotubes (CNTs), nanoparticles of silver (AgNPs), gold (AuNPs), platinum (PtNPs), zinc oxide (ZnONPs), cadmium sulphide (CdSNPs), titanium dioxide (TiO2NPS), iron oxide (IONPs), copper (CuNPs), cerium oxide (CeO2NPs), silicon (SiO2NPs) and dendrimers in cell as well as animal models.
In vitro and in vivo studies show that these NPs elicit specific effects on serum enzymes, inflammatory cytokines, oxidative stress, gene expression and morphology of hepatocytes. Antioxidants like vitamin C and α lipoic acid can reverse the cell injury in some cases. Further, protective effects of ZnONPs and CeO2NPs against experimental hepato-carcinogenesis have also been highlighted. It is suggested that further efforts are required to address health issues concerned with nanomaterials.
Liver, Nanoparticles, Carbon nanotubes, Quantum dots, Dendrimers, Cell death
NPs: Nanoparticles; RES: Reticuloendothelial system; QDs: Quantum dots; ROS: Reactive oxygen species; LPO: Lipid peroxidation; CNTs: Carbon nanotubes; AgNPs: Silver nanoparticles; AuNPs: Gold nanoparticles; ZnONPs: Zinc oxide nanoparticles; PtNPs: Platinum nanoparticles; CdSNPs: Cadmium sulphide nanoparticles; TiO2NPs: Titanium dioxide nanoparticles; IONPs: Iron oxide nanoparticles; CNPs: Copper nanoparticles; CeO2NPs: Cerium oxide nanoparticles; SiO2NPs: Silicon nanoparticles; CCl4: Carbontetrachloride; APAP: Acetaminophen; PAMAM: Poly amidoamine; ALT: Alanine aminotransferase; AST: Aspartate transaminase; LDH: Lactate dehydrogenase; GPx: Glutathione peroxidise; GSH: Reduced glutathione; GA: Glycyrrhizic acid; SD: Sprague Dawley; BBB: Blood brain barrier
It was during the meeting of American Physical Society (1959) when Richard. P. Feynman asked, "What would happen if we could arrange the atoms one by one the way we want them"? This meeting gave birth to the concept of nanotechnology. The word nano is linked to a Greek term "nanos" meaning dwarf. Today, nano is popular label in science, technology and medicine. During 1990-2000, new processes for the synthesis of nanoparticles (NPs) were developed. In subsequent years, health hazards posed by NPs were recognized. NPs have emerged as a new class of environmental pollutants that may affect atmosphere, terrestrial and aquatic ecosystems. Their prevalence in ecosystems can be harmful to human beings, animals, plants and aquatic species [1]. Furthermore, occupational exposure to NPs during their production, commercial and biomedical use may lead different health problems and safety issues [2].
The science that mainly addresses safety issues of NPs is known as nanotoxicology. It refers to the studies on interaction between nanostructures and biological systems. Specific responses are elicited by NPs corresponding to their size, shape, composition, surface chemistry and aggregation. Nanoparticles have been classified into two groups i.e. engineered NPs and incidental NPs [3]. Quantam dots, carbon nanotubes, dendrimers and fullerenes which have a diameter < 100 nm are known as engineered NPs (ENPs). Whereas, accidentally generated diesel particles are called incidental NPs. They can enter human body through different portals i.e. dermal, respiratory, gastro-intestinal, ocular, auditory, intravenous and mucous routes. Their toxicity is determined by barrier function and clearance mechanisms at respective portals of entry. Large surface area to volume ratio facilitates interactions between cell membrane and NPs [4]. Modifications in NPs surface may cause undesirable ionic interactions with biological systems [5].
To-date, limited information is available on the absorption and translocation of NPs. Experimental studies made in rodents have demonstrated that NPs deposited in lungs can translocate to the pulmonary interstitium [6]. Translocation of NPs from lungs to secondary organs i.e. liver, kidney, heart and brain depends on their physical properties [7]. Translocation of inhaled NPs to brain has been associated with neurodegenerative diseases caused by air pollutants [8]. Nevertheless, skin is potent barrier to certain nanomaterials. Similarly, NPs undergo limited gastrointestinal absorption except in environmental or occupational exposures. Smaller, hydrophobic and neutral particles are prone to increased absorption.
Skin, pulmonary and reticuloendothelial system (RES) including liver and spleen have been identified as the main target organs for NPs toxicity. NPs are taken over by RES through opsonisation [9]. Aside RES, kidney may be another organ of NP toxicity. Some fullerenes and dendrimers have been known to distribute in renal tissue [10,11]. There are many examples of particulate induced lysosomal dysfunction. Alterations in lysosomal permeability and subsequent release of lysosomal enzymes contributed to apoptosis induced by silica microparticles in alveolar macrophages [12]. Nanoparticles of neodymium oxide [13]; quantam dots [14] and fullerenes [15] are also known to induce autophagy in vitro.
Size, shape and surface charge determine the uptake of NPs by cells through size selectivity matching their endocytic pits. Absorption of NP may occur through phagocytosis or pinocytosis. Pinocytosis is further classified as macropinocytosis where particles > 1 μm are absorbed. Other mechanisms are clathrin or caveolae mediated endocytosis or clathrin-caveolae independent endocytosis. Caveolae are made by plasma membrane invaginations of 50-80 nm size containing cholesterol and sphingolipid receptors [16,17]. Endocytosis may occur through lipid rafts that offer suitable platforms to facilitate assembly of receptors, adaptors, regulators and other downstream proteins as a signalling complex [18].
Similarly, clathrin coated pits of 100-200 nm have been shown to be associated with scaffold proteins such as AP-2 and eps-15 [19]. Nevertheless, the specific endocytic mechanism by which cells internalize specific NPs such as quantam dots remains unknown (Figure 1) summarizes some of these mechanisms that may occur in a macrophage or epithelial cell.
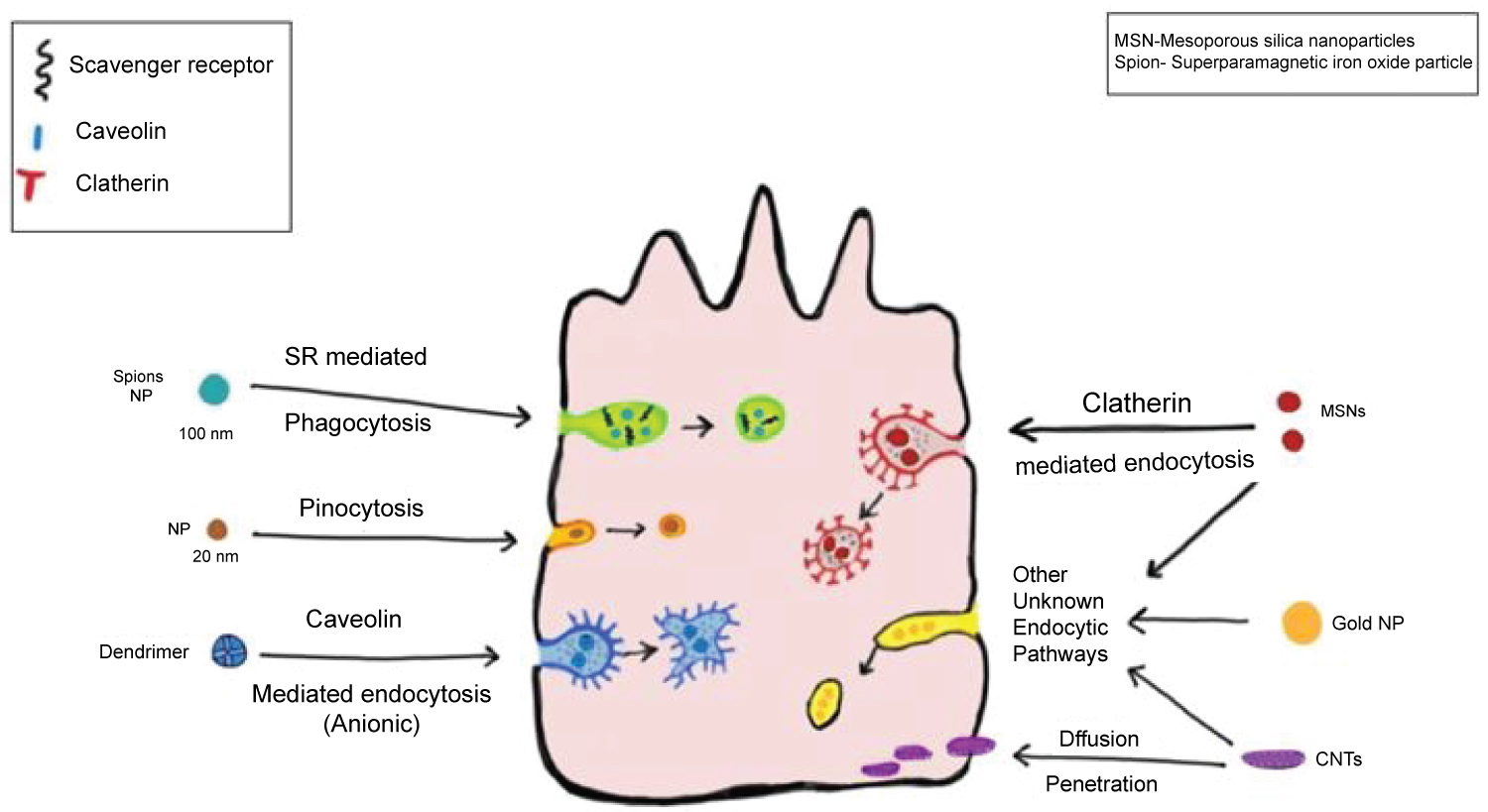 Figure 1: Cellular uptake of nanoparticles by a macrophage/epithelial cell.
View Figure 1
Figure 1: Cellular uptake of nanoparticles by a macrophage/epithelial cell.
View Figure 1
The size and shape of NPs is different from bulk particles. Therefore, their interaction with biomolecules i.e. proteins and lipids has been found to be different. They can bind with proteins and form a protein corona. CdSe/ZnS Qds formed a 3.3 nm thick corona with human serum albumin [20]. Further protein binding depends on the type of cell/system and the medium used. The interaction of NPs with biomolecules undergoes conformational changes [21,22]. These conformational changes determine cellular health as the immune system may treat them as foreign object and may try to eliminate them. Protein-NP interaction may allow their escape from endocytic route and promote cytotoxicity [23]. NPs can enter nucleus in some cases. In in vitro settings, they can cross the placental barrier [24,25]. Their accumulation in subcellular structures i.e. endosomes is higher than the free ions. NPs can directly interact with lipids. They can adhere to membranous lipids causing disturbances in membrane fluidity. It facilitates the penetration of NPs into the cell bypassing the endocytic route [23].
NPs could cause adverse cellular and molecular effects through several mechanisms. These include generation of reactive oxygen species (ROS). Cytoskeletal effects. Intracellular signalling pathways and genotoxicity. NPs can generate ROS through different mechanisms. It can occur through direct effect as a result of exposure to acidic environment or through leached ions [26,27]. Their effects on mitochondria may cause the generation of ROS [28]. Interaction of NPs with redox active proteins such as NADPH oxidase and cell surface receptors may also lead to lipid peroxidation (LPO) [29]). However, kinetics of ROS induction can differ between various NPs.
Intracellular localization of NPs has been known to disrupt the cytoskeleton network. Effects of QDs on cytoskeleton have scarcely been studied. No differences in pheochromocytoma cells incubated with CdTe QDs for 72 h were reported [30]. However, significant structural changes in actin and tubulin networks of 3T3 fibroblasts after incubation with CdSe/ZnSe QDs were demonstrated [31]. Different surface modifications were also found to cause different degrees of cellular effects [32]. Moreover, gold NPs affected the morphology of several cell types such as A 549 human carcinoma lung cells [33]. Secondary effects caused by cytoskeletal changes are yet to be established.
NPs can interfere with delicate balance of cellular homeostasis and thus alter complex intracellular signalling pathways. Possible effects may be genotoxic caused by high levels of ROS [34]. Protein or gene expression may be altered due to perinuclear localization of NPs that may affect the transcription and translation functions [35]. Leaching of metal ions may also modulate protein or gene expression [36]. NPs may also interfere with cell surface receptors [37]. Specific effects of NPs on liver are discussed in following paragraphs.
Carbon nanotubes were first discovered by Diego and coworkers [38]. They are allotropic modifications of carbon, represented as a sheet of graphene (single layer of graphite) rolled into a cylinder. They are further classified as single walled or SWCNT or multi walled or MWCNT [39,40]. They are used in numerous technological applications including novel drug delivery systems [41]. They can interact with macromolecules such as proteins and DNA [42]. Several workers have investigated their toxicity in different systems. Multiwall carbon nanotubes induced oxidative stress and cytotoxicity in human embryonic kidney (HEK 293) cells [43]. SWCNTs also increased malondialdehyde and decreased GSH level in mice [44]. Only a few reports are available on the hepatotoxicity of CNTs. However, cytotoxicity of SWCNTs on hepatoma HG2 cells has been reported [45]. A dose-effect relationship on the effects of MWCNTs was reported in the liver of Kunming mice exposed to 10 and 60 mg/kg MWCNT [46]. They reported an increase in total bilirubin and AST in MWCNT treated rats as compared to PBS treated rats. Gene expression studies showed changes in G protein coupled receptors, cholesterol synthesis, CYP450, TNFα and NFkB signalling pathways. An interesting study has shown that rats exposed to SWCNT through intratracheal installation exhibited NMR based metabonomic changes in the blood plasma and liver extracts [47]. It has also been hypothesized that CNTs directly induce ROS generation that caused oxidative stress to various cells through inflammation and apoptosis [48]. Combined hepatotoxic effects of MWCNT and cadmium (Cd) were studied in mice [49]. These researchers showed that MWCNT reduced hepatotoxicity of Cd. These observations were based on their findings on serum transaminases, total bilirubin and blood urea nitrogen (BUN). They speculated the role of metallothionein (MT) in MWCNT toxicity.
The structure of quantum dots has been studied by several workers [50,51]. These are special nanocrystals ranging from 1 to 10 nm in diameter. They exhibit unique electronic, optical, magnetic and catalytic properties. QDs show immunotoxiciyty and can induce oxidative stress and DNA damage [52]. It has also been demonstrated that QDs can cause cell death by lipid peroxidation of human neuroblastoma cells [53]. Hepatotoxicity of QDs in the liver of man and animals has also been studied by a few workers. These results are summarized in the following paragraphs.
Silver from ancient times has been used as a therapeutic element. However, silver nanoparticles have been used for their antimicrobial activity. Very few workers have studied the cytotoxicity of AgNPs. The effects of different dose regimen on hepatotoxicity of AgNPs in rat have been studied by a few researchers [54]. They performed liver function tests and recorded the bioaccumulation of AgNPs in liver. Though elevated values for ALP were observed, other parameters showed no significant changes in comparison to controls.
Contrarily, hepatoprotective effects of AgNPs against CCl4 induced hepatotoxicity were observed [55]. Authors used AgNPs synthesized using aqueous leaf extract of a mangrove based plant Rhizophora apiculata. In other reports, edema and necrosis in the liver of male ICR mice after a single intravenous (8 μmol/kg) injection of AgSe (5.1 nm) QDs were observed [56,57]. However, mechanisms of cytotoxicity expressed by AgNPs have not yet been established. Silver ions released from medicaments may enter circulation facilitating translocation and accumulation in soft organs like liver and kidney [58].
Effects of two dose regimen i.e. 20 and 50 ppm (14 days) of silver nanoparticles on liver function of BALB/C mice were also studied [59]. These workers noticed that level of serum transaminases viz: alanine aminotransaminase (ALT) and aspartate transaminase (AST) increased significantly in AgNP treated mice. No significant difference was noted in male and female mice.
A study made on approximately 8.7 nm silver nanoparticles administered in albino rats for 28 days at 1, 2, 4 mg/kg b.w. concluded that AgNps induced hepatotoxicity through oxidative stress mechanisms. Bioaccumulation of AgNPs in liver and chromosomal aberrations in bone marrow was also recorded [60]. Oxidative stress induced by AgNPs was found to be a dose dependent phenomenon. Administration of 10 nm citrate stabilized AgNPs at 0.2 mg/kg b.w. to male Wistar rats for 14 days induced mild oxidative stress in brain but not in the liver [61]. A few researchers have attributed AgNPs toxicity to its preferential accumulation in liver. AgNPs in combination with copper and boron as a composite in the dose range of 1-20 mg/kg on early (4-24 h) acute exposure and late phase (96 h) exposure in normal and NLRP-3 deficient mice were found to cause acute liver injury. Elevated values for ALT, AST and LDH along with necrosis, Kupffer cell hyperplasia and lobular granulomas were observed [62].
Developmental hepatotoxicity of AgNPs has also been investigated by a few researchers. Post-gestational (1-19 days) administration of AgNPs (25 mg/kg) through intra-gastric gavage induced oxidative stress in the liver of rat pups. While glutathione peroxidise (GPx) activity and reduced glutathione (GSH) levels were decreased. Malondialdehyde and caspase-9 levels were significantly increased. Histological studies exhibited fatty degeneration [63].
Although antimicrobial effects of silver nanoparticles are well known, it was found to protect against acetaminophen induced hepatotoxicity. Rats were treated with three different dosages of AgNPs (50, 100, 150 μg/kg p.o.) after APAP treatment (2 g/kg p.o. once only). Serum enzymes values and bilirubin level declined after AgNP treatment. This report further suggests a therapeutic value of AgNPs [64].
Recently, a few authors have studied the molecular toxicity of AgNPs on liver microsomal fraction. NPs (5-80 nm) were administered daily to growing Wistar rat for 92 days. Electrophoretic studies revealed the presence of proteosome activator complex (Psme 1) and heat shock protein (HSPd 1) gene. AgNP treatment caused the disappearance of protein of B-2α tubulin chain (tuba 1b gene) from the microsomal fractions [65]. Molecular aspects of AgNPs toxicity along with ultrastructural changes were reported by a group from Thailand. Microarray study revealed up and down regulation of those genes that were not up or down regulated by Ag ion exposed cells. Hep G2 cells in AgNP treated group showed distorted ultrastructural changes [66].
The use of AuNPs in nanomedicine has been suggested by a few scientists. Contrarily, a few authors found them to be toxic. Thus biosafety issues related with AuNPs have raised concerns for human health. A few researchers following standard procedures/protocols have confirmed its hepatotoxic effects. Healthy and damaged liver of mice showed differential effects of AuNPs in mice. Hwang, et al. [67] first induced liver injury in mice by feeding them with methionine choline deficient diet and then subjected them to AuNPs treatment. They recorded higher values for ALT, AST and ROS in the mice. They concluded that AuNPs display toxicity in stressed liver. Another study from Reshi and coauthors contradicted these results [68]. They showed that AuNPs ameliorate APAP induced hepatotoxicity in albino rats. They considered AuNPs as potential hepatoprotective agents. It has been demonstrated that intraperitoneal administration of AuNPs induces liver damage through oxidative stress. These effects are alleviated by melanin (an antioxidant) treatment [69].
Coating AuNPs with suitable carrier molecules was also found to affect their toxicity. Polyethylene glycol (PEG) coated AuNPs were less toxic than uncoated particles. These observations were made based on the results on serum transaminases and histopathological findings [70]. Coating of AuNPs with citrate and chitosan also affected the hepatotoxicity of AuNPs. Chitosan capped NPs were less toxic than citrate capped ones in Swiss mice. This observation was made by after analysing the inflammation related genes using RT-PCR [71].
Platinum nanoparticles (PtNPs) are widely used in cosmetics, industry and diagnostics. On absorption, nanoplatinum can accumulate in soft tissues viz: liver, spleen, kidney, lungs and heart. A study from Poland recently reported that PtNPs can induce DNA damage and apoptosis in liver [72]. Dose dependent hepato and renotoxicity of PtNPs has also been demonstrated. NP of 1 nm diameter when administered with CCl4 or cisplastin induced hepatotoxicity whereas those of 8 nm did not exaberate the toxicity in mice [73]. Toxic effects of subnanosized platinum particles (snPt) in mouse liver were also studied. Increased levels of inflammatory cytokines and histopathological changes in liver of mice were observed after intravenous administration of snPt at 15 mg/kg body weight. However, administration of nanosized platinum particles did not produce these abnormalities [74].
Nanoparticles of zinc are better known for their hepatoprotective than hepatotoxic effects. Zinc oxide nanoparticles (ZnOPs) are widely used in cosmetics, sunscreens, clothes,medicine and electronic devices. Several studies demonstrate that ZnOPs induce oxidative stress and apoptosis in hepatocytes [75]. Furthermore, a study showed that ZnOPs at 200 mg/kg and 400 mg/kg given through gavage to mice for 90 days induced focal necrosis and increase in AST and ALT values [76]. In addition, mRNA expression level and ER stress related genes (grp78, grp74, pdi-3, xbp-1) were also upregulated. These workers reported upregulation of ER stress associated apoptotic protein levels viz: caspase-3, caspase-9 and caspase-12.
Hepatotoxicity of Mn doped ZnS nanoparticles in mice were also studied by the same group of researchers [77]. They estimated ALT, AST, catalase, glutathione peroxidise, superoxide dismutase and malondialdehyde in the liver of Mn doped ZnSNP treated mice. Mn doped ZnSNP did not cause any obvious damage to liver. Contribution of QDs in hepatocyte pyroptosis and inflammation has also been investigated. QDs expressed cytotoxicity in LO2 cells in a dose dependent manner [78]. QDs activate NLP pyrin domain containing 3 (NLRP3) inflammosome in hepatocytes leading to a novel pro-inflammatory form of cell death called pyroptosis. NLRP3 activation was caused by QDs triggered mtROS production and Ca(2+) mobilization. In another study, ZnONPs at a daily dose of 2 mg/kg for 21 days administered to male Wistar rats induced structural changes in the liver. Sinusoidal dialatation, Kupffer cell hyperplasia, inflammatory cells infiltration, necrosis, hydropic degeneration, apoptosis, karyolysis, glycogen depletion and haemosiderosis were reported [79].
It was postulated that ZnONPs offered protection against dimethylnitrosamine induced hepatotoxicity in rat by inhibiting oxidative stress. Moreover values of proinflammatory cytokines viz: TNF-α and IL-12 were also reduced [75].
A comparison of hepatotoxic effects of zinc nanoparticles and zinc oxide nanoparticles in rat has also been made. Intraperitoneal administration of these particles increased the activity of gamma glutamyl transferase (GGT), lactate dehydrogenase (LDH) and caspase-3. Induction of TNF-α was also registered. They concluded that zinc nanoparticles are less toxic than ZnONPS [80].
Hepatotoxicity of cadmium microparticles on liver and other organs of man and experimental animals have been studied in the past by several laboratories. However, toxicity of cadmium nanoparticles is poorly known. It was shown that Cd/Se/Te based quantam dots 705 modulated liver redox balance in mice [81]. A dose dependent increase in metallothionein expression and liver function impairment was noted. Further a corresponding increase in oxidative stress, oxidative DNA damage and inflammation was noticed. QDs activated NLRP3 and pyroptosis. It was attributed to mitochondrial ROS and Ca2+ mobilization [82]. Hepatotoxicity of Cds nanoparticles of different size/length was also studied [78]. It was interesting to note that hepatotoxicity of smaller CdSNP was greater than the larger CdS NPs.
Titanium dioxide nanoparticles (TiO2NPs) are now widely used in food, cosmetics, agriculture, medical devices and building engineering related processes. Their effects on human health have recently been reviewed. Liver disorders caused by TiO2NP were reported to increase the level of serum trans-aminases, LPO and oxidative stress in Wistar rats treated with 300 mg/kg of TiO2NP for 14 days by gavage [83]. These researchers showed that glycyrrhizic acid (GA) protected the rats against hepatic injury caused by TiO2NP. In vitro and in vivo toxicity of TiO2NP enhanced under oxidative stress conditions. A study made on BRL-3A liver cells and in the liver of Sprague-Dawley (SD) rat suggested a synergy between oxidative stress and TiO2NP induced hepato-toxicity [84]. Further cell death ratio was significantly enhanced (up to 2.62 fold) in BRL-3A cells exposed to OS and TiO2NP. A comparative study on the hepatotoxicity of TiO2NPs and sodium oleate coated iron oxide nanoparticles (OC-Fe3O4NPs) was made in Wistar rat [85]. Based on results on redox defences, these researchers concluded that OC-Fe3O4NPs do affect redox enzymes but liver is able to retain its functional integrity. TiO2NPs were also found to affect metabolic function of liver. In a study made in mice treated with TiO2NP (21 nm) for 14 days, ultrastructural changes viz: Mitochondrial oedema and gene expression variations were noticed [86].
A few studies have been made to investigate the reversal of TiO2NP toxicity by antioxidants. Oxidative stress induced by TiO2NPs and TiO2 bulk particles (150 mg/kg) in the liver of Sprague-Dawley rats was reversed by extracts of a common herb cinnamon (Cinnamonium cassia). Morphological as well as haematological improvements were also recorded [87]. In another study, tiron was also found to protect against the toxicity of TiO2NPs. While TiO2NPs upregulated the proapoptotic Bax gene and down regulated the antiapoptotic Bcl-2 gene, tiron upregulated Bcl-2 and decreased Bax expression. These results were supported by observations on serum enzymes and histopathological changes [87]. Moreover, quercetin and idebenone also ameliorated hepatotoxicity caused by TiO2NPs. These antioxidants modulated serum enzymes, VEGF, NO, DNA damage in the liver of TiO2NP treated rat liver [88].
Protective role of vitamin A and vitamin E against liver injury caused by TiO2NPs in male Wistar rat has also been determined. Enzyme biomarkers of liver function, histopathological changes, antioxidant enzymes and inflammatory mediators were increased in TiO2NPs (300 mg/kg) treated rats. Vitamin A and E both inhibited these parameters showing an ameliorative effect [89].
Iron oxide nanoparticles are used in different disciplines of diagnostic science, biomedical sciences and drug delivery systems. On penetration, IONPs are taken up by cell organelle (endosomes/lysosomes) especially in hepatocytes. They contribute to cellular iron pool and release into cytoplasm after decomposition. Magnetic iron oxide nanoparticles can accumulate in liver, spleen, lungs and brain after inhalation. It shows their ability to cross the BBB [90]. Enzyme biomarkers of liver function viz: AST, ALT, and LDH increased in the liver of rats treated with different concentration of IONPs. In IONPs exposed rats at three concentrations i.e. 500, 1000, 2000 mg/kg, an increase in serum transaminases at all concentrations was recorded [91]. Whereas, exposure of rats to bulk iron oxide exhibited no effect. Another study reported that a dose of 10 mg/kg of dextran coated IONPs do not affect functional integrity of liver in Wistar rat [92]. A few studies have been made to observe the effects of superparamagnetic nanoparticles (USP IO-NPs) on LO2 cells [93]. These particles could induce cytotoxicity and caused leakage of LDH. These particles affected several genes especially those related with calcium homeostasis and inflammation i.e. IL, 1B, IL6 and IL8. USP IONPs can cause ER stress as well. ER stress induced by several nanoparticles has recently been discussed by Rana [94]. NPs have been shown to modulate the cross talk between mitochondria and ER. Human hepatic L02 cells when exposed to USPIO- NPS (2.5, 7.5 and 12.5 μg/mL) for 6 hr did not cause hepatic injury. However, it induced apoptosis at 20 μg/mL after 24 hr. It was postulated that the injury is mediated by Cox-2 genes [95].
Copper nanoparticles (CNPs) work as anti-biotic, anti-microbial and antifungal agents when added to plastic items and textiles. They are used in conductive inks and pastes as a substitute for expensive metals in electronic displays and transmissive conductive thin film applications. Toxicity of CNPs is not fully understood. However, available data show that CNPs at a high dose (200 mg/kg/d for 5 days) could induce overt hepatotoxicity in rats. An integrated metabolomic study revealed mitochondrial failure, enhanced ketogenesis, fatty acid β oxidation and glycolysis to contribute in its toxicity [96]. High dose(s) of CNPs could elevate serum enzymes, triglytcerides and bilirubin. Histopathological observations exhibited necrosis. Further, gene expression studies indicated that genes related to oxidoreductases, metabolism and signal transduction pathways were involved in the development of hepatotoxicity [97]. CNPs have been found to be toxic in fish also. A fresh water fish, Cyprinus carpio, when exposed to CNPs and CuO at 0.5, 1.0 and 1.5 mg/L showed higher values for malondialdehyde in the liver. It was concluded that LPO contributes in the hepatotoxicity of CNPs [98].
Amelioration of CNPs induced hepatotoxicity by extracts of green tea has also been reported [99]. Theses researchers concluded that green tea offered protection against hepatotoxicity of CNPs (20-30 nm) administered at a dose of 40 mg/kg/b w. Green tea improved the activity of liver enzymes, antioxidant status and suppressed DNA fragmentation and the expression of caspase-3 and Bax proteins. Another agent that offered protection against CNPs induced liver damage was α lipoic acid [100]. Status of LPO, NO, copper and apoptotic genes (c-myc and c-jun) improved in CNPs treated rats when co-adminstered with α lipoic acid. These results suggest that CNPs induce hepatotoxicity through oxidative stress in rat.
Cerium is the second member and the most reactive element in the lanthanide series. Cerium oxide (CeO2/ceria) is considered the most suitable oxide of cerium. Ceria nanoparticles demonstrate the formation of more oxygen vacancies. The large surface area to volume ratio in its nanoparticle enables CeO2 to react differently resulting in unique properties.
Nanoceria is used in solid oxide fuel cells, catalytic applications and photocatalysis. Recently it has drawn considerable attention as a therapeutic agent in the treatment and prevention of diseases associated with oxidative stress. A few reports are available on its hepato-protective rather than hepato-toxic effects. D-galactosamine and lipo-polysaccharide induced hepatotoxicity in rat was alleviated by CeO2 [101]. These workers showed that CeO2NP decreased translocation of cytoplasmic Nrf-2 with concomitant decrease in gene expression of HO-1. These effects were attributed to its antioxidative properties. Results on antioxidant enzymes and histopathological observations supported this conclusion. Another evidence of antioxidative potential of CeO2 is provided by experiments made on monocrotaline induced hepatotoxicity in mice [102]. It was shown that monocotaline induced decrease in hepatic GSH, GPx, GR and GST is normalized after the treatment of CeO2NPs.
A recent study showed that CeO2NP offered protection against diethylnitrosamine induced hepatotoxicity in mice. Pretreatment of CeO2NPs attenuated the activity of antioxidant enzymes and expression of Bcl2 and Cox2. This report again supports the antioxidative role of CeO2NPs [103].
Silica nanoparticles are employed in several commercial, agriculture and medical applications. However, the information on their health effects remains elusive. A few studies are available on their effects on structure and function of liver. Size of silica nanoparticles seems to be a confounding factor in their toxicity. It was reported that silica particles having a diameter of 300 to 1000 nm elicited no adverse effects while SP-70 could induce liver injury at 30 mg/kg b.w. [104]. Repeated administration of SP-70 twice a week for four weeks at 10 mg/kg b .w. caused hepatic fibrosis. Serum enzyme markers were also increased. Hasezaki, et al. also confirmed that SP-70 is potent hepato-toxin [105]. Silica nanoparticles (14 nm) were found to induce apoptosis in human liver (HepG2) cells that was regulated by ROS through p53, bax/bcl-2 and caspase pathways. ROS scavenger, vitamin C modulated apoptotic markers [106].
There are reports suggesting that SiO2NPs (10-80) when administered to rats (nm) disturb tricarboxylic cycle and liver metabolism. It can induce oxidative stress and alter liver cell morphology (150 μg) for 90 days [107]. Another study supported these results through global metabolomic study on SiNPs treated human hepatoma cells (HepG2) and ICR mice liver. This study concluded that glutathione metabolism and oxidative stress are amongst the principal causes of SiNP induced hepatotoxicity [108]. SiNPs influence CYP450 both in rat and human hepatocytes [109]. In addition to apoptosis, SiNPs can induce pyroptosis through NLRP3 inflammasome activation which is caused by mesosporous silica nanoparticles (MSN) induced ROS generation [110]. A recent study suggested that SiONPs (10 nm) make profound changes in morphometry, biochemistry, hematology and genes expression of DMES in rat [111].
The term dendrimer comes from a Greek word meaning "Dendron". Dendron is translated into a tree. A dendrimer possesses a symmetric structure around a core. A dendrimer molecule has hundreds of possible sites to couple to an active species. It possesses a hydrophobic core and hydrophilic periphery. It exhibits a micelle like behaviour. Dendrimers have been explored for encapsulation/scaffolding of hydrophobic compounds and anticancer drugs.
There are certain anticancer drugs i.e. methotrexate and 6-mercaptopurine that exhibit hepatotoxicity. When these drugs were encapsulated by the dendrimer based melamine and administered in C3H mice at subchronic doses, significant reduction in hepatotoxicity was observed [112]. Subsequently, the same group of researchers showed that melamine dendrimer given at 40 mg/kg to mice resulted into hepatotoxicity as determined through serum enzymes and histopathological changes [113]. Role of their route of administration was also discussed [114]. It was interesting to note that the nanomaterials such as poly-amidoamine (PAMAM) that are widely used in pharmaceutical industry caused hepatotoxicity through growth inhibition, mitochondrial injury and apoptosis in human liver cells. Blockage of autophagy in PAMAM treated mice led to hepatoprotection [115].
In recent times, nanoparticles have emerged as a new class of environmental pollutants. Additionally, occupational exposure to NPs may contribute to some unknown health issues in humans. Their toxicity is determined by barrier functions and clearance mechanisms at respective portals of entry. Experimental studies demonstrate their adverse effects on skin, lungs, liver, kidney, heart and brain of experimental animals. The interaction between NPs and biomolecules causes conformational changes that determine cellular health. The adverse effects are categorized as autophagy, apoptosis, necrosis, pyroptosis, oxidative stress, cytoskeleton changes and altered intracellular signalling pathways. Change in gene and protein expression affects transcription and translation functions.
Present review summarizes the specific hepatotoxic effects of ENPs. To note, CNTs can directly induce oxidative stress, inflammation and apoptosis, whereas, silver NPs could cause edema and necrosis. They exhibit protective effects against liver injury caused by CCl4 and acetaminophen. It is interesting to note that subnanosized platinum nanoparticles cause increased secretion of inflammatory cytokines. Smaller CdSNPs induce greater hepatotoxicity than larger NPs. Earlier studies demonstrated TiO2NPs affect metabolic function of liver. IONPs were found to cause ER stress. Copper nanoparticles can cause mitochondrial failure. However, CeO2NPs offer protection against dimethylnitrosamine toxicity. Available reports indicate that redox imbalance is the principal cause of SiNP induced hepatotoxicity. Dendrimers do affect growth inhibition, mitochondrial function and induce apoptosis.
These observations suggest that there appears an urgent need to develop nanosafety research. In vitro studies on NP toxicity on new models like hepatocyte like cells derived from puripotent stem cells using toxicogenomic tools have opened new avenues in NP research [116]. Further research on molecular toxicology of NPs as well as on their therapeutic values is warranted. Defining the interactive mechanism between NPs and biological molecules will be helpful in designing safer nanomaterials.
The author declares no conflict of interest in the preparation of this article.
Financial assistance received from Indian Science Congress Association in the form of Sir Asutosh Mookerjee Fellowship to the author is gratefully acknowledged.