Pollution by heavy metals in aquatic ecosystems has become the central focus of environmental research, due to the threat it poses to consumers of fishery sources. This study seeks to assess the levels of accumulation and potential human risk associated with heavy metals in Chrysichthys nigrodigitatus from Ologe and Badagry lagoons Southwestern Nigeria. The metals (Cr, Cd, Fe, Ni, Cu, Zn, and Pb) in fish samples were analyzed with atomic absorption spectrometer (AAS), while the estimated daily intake (EDI), target hazard quotient (THQ) and target cancer risk (TR) were also estimated. All metals in the muscles of C. nigrodigitatus were below the WHO/FMEnv guideline. Pearson correlation coefficient of heavy metals in muscle of C. nigrodigitatus showed significant correlation (p < 0.05) between Cu and Pb (r = 0.990*), Cr and Cu (r = 0.962*), Cr and Pb (r = 0.985*). The target hazard quotient (THQ) of the metal decreased in the following order: Pb > Zn > Cd > Fe > Cu > Cr > Ni and the hazard index (HI) risk values were 0.203 and 0.140 at Ologe and Badagry lagoons respectively. The THQ for all heavy metals estimated across both lagoons were all less than 1. The target cancer risk (TR) observed the highest values of 4.3 × 10-5and 5.20 × 10-5for Ni at both Ologe and Badagry lagoons respectively. The THQ had no adverse health effects from the consumption of fish. However, target cancer risk (TR) due to Pb and Ni exposure through fish consumption may increase the probability of developing cancer in the future. The biomonitoring of metal accumulation in tissue of Chrysichthys nigrodigitatus underscores the need for periodic monitoring of the lagoon.
Heavy metal, Risk assessment, Fish, Lagoons
Fish consumption serves as one of the main entries through which aquatic pollutants such as heavy metals are bioaccumulated and biomagnified along the food chain, in other words, to ascertain the human health implications of ingesting fishery resources contaminated with heavy metals; risk assessment tool has been employed to estimate the associated risk as food safety measures as well as understanding the pollution status of the aquatic environment.
In recent years, fish contamination by heavy metals and other organic compounds in aquatic ecosystems has become the central focus of environmental research due to the threat it poses to consumers of fishery sources. Heavy metals constitute a core group of aquatic pollutants via its bio-accumulative and non-biodegradable properties in food [1]. More attention should be given to toxic heavy elements due to bioaccumulation and biomagnification potential by human, associated with aquatic systems by consumption of contaminated fish and other aquatic foods from this environment. Fish are sometimes categorized at the higher level of the aquatic food chain; adequate concentration of these metals may bioaccumulate in the organs of the fish species and biomagnified in humans via consumption.
Essential elements such as iron (Fe), copper (Cu) and zinc (Zn) are required in biological systems for necessary functions as growth. Within the systems, higher concentrations of these essential metals via continuous deposition of discharges from anthropogenic activities further enhance the levels which become toxic to the biota and environments [2]. Furthermore, the non-essential metals such as nickel (Ni), cadmium (Cd), chromium (Cr) and lead (Pb) are highly toxic at trace amounts in any biological systems ranging from aquatic biotato human along the food chain. The accumulation of metal serves as a tool towards identifying the impact of metal in the aquatic ecosystem and therefore shows an adverse effect in an organism [3]. The amount of metal in fish is dependent on the concentration levels of these metals in the food and their habitats as well as the detoxification rate of the metals. When metals enter into the environment, they may accumulate in the food chain and cause serious ecological damage and also pose carcinogenic and other adverse effects on human health due to biomagnification over time [4].
Human health risk assessments of heavy metals in fishery resources focus on the characterization of the potential adverse health effects of human exposure to environmental hazards [5]. Risk assessment serves as a tool in the assessment, identification, and measurements of hazards, determine possible pathways of exposure, and eventually utilize the acquired data to estimate the potential risk associated with consuming the exposed organism. The human health risk assessment involves four; 4) Tasks which involves; (a) Hazard identification, (b) Dose-response assessment, (c) Exposure assessment, and (d) Risk characterization [6].
Health risk assessment classifies elements as non-carcinogenic or carcinogenic. The non-carcinogenic chemicals are assumed to have a threshold limit and as such are considered with no adverse health effects at a dose below the threshold level estimated with the dose-response assessment method calculated with the reference dose (RfD) of the specific element. While carcinogenic chemicals are assumed to have no effective threshold limits. This assumption indicates that the risk of cancer developing over time with exposures to the chemicals is at low doses. Thus, there is no safe threshold for exposure to carcinogenic chemicals and are expressed by their Cancer Potency Factor [6].
Lagoons lies within the southwestern region of Nigeria, which serves to contribute immensely to the socio-economic growth such as recreational, fishing, sand dredging, and aquaculture activities. Adequate numbers of industries are sited along these coastal regions, which contribute discharge of effluents into the ecosystems. Therefore, the health risk assessment of non-carcinogenic and carcinogenic chemicals was used in this study as a tool to assess, identify, measure and quantify the health risk implication of heavy metals from direct and indirect sources of pollution in coastal ecosystem based on the food chain relationships. Thus, this study seeks to determine the levels of accumulation and potential human risk assessment of heavy metals in Chrysichthys nigrodigitatus from Ologe and Badagry lagoons Southwestern Nigeria.
Ologe and Badagry lagoons constitute sections of the southwestern lagoons in Lagos State (Figure 1). Ologe lagoon is a freshwater environment all year round, while Badagry lagoon is usually partly fresh during the dry season and brackish during the wet season as it receives the influx of seawater via the Porto Novo Creek, Benin Republic. These lagoons are usually dominated with aquatic invasive weed such as water hyacinth which proliferates and impedes transportation with boats from fishing activities. The mangroves at the lagoons consist of Avicennia nitida, Laguncularia racemosa, Rhizophora racemosa and other ferns such as Raphia palms.
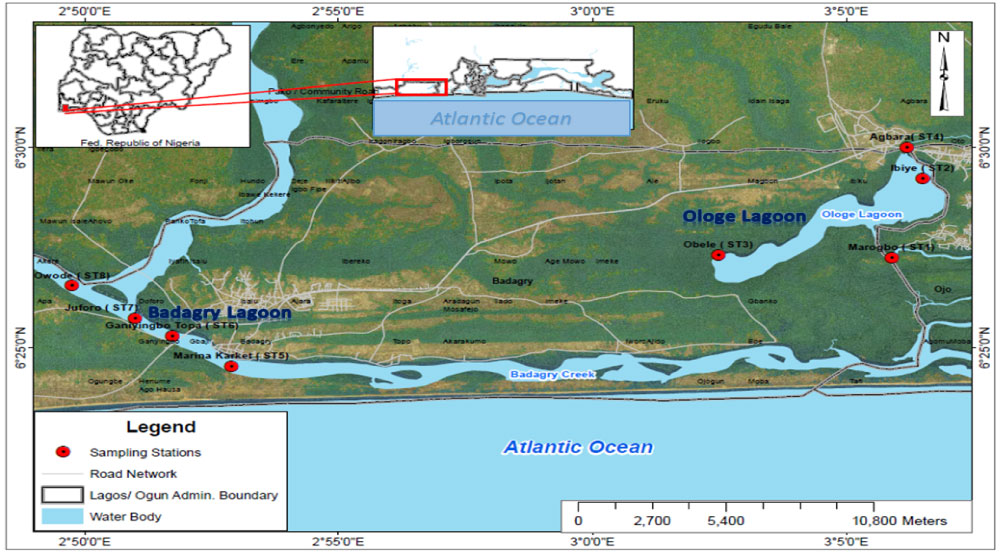 Figure 1: Sampling stations at Ologe and Badagry Lagoons.
View Figure 1
Figure 1: Sampling stations at Ologe and Badagry Lagoons.
View Figure 1
A total of 192 fish samples (Chrysichthys nigrodigitatus, 96 adults from each lagoon)were caught with trap basket by local fisher folks set at the bottom of the lagoons, due to the habitat of the fish species been bottom dwellers (demersal). The fish samples were frozen and stored at -18 ℃ immediately after and transported to the laboratory for digestion procedures and analysis. Five grams (5 g) weighed muscle sample was excised from the fish and the ashing was done at 500 ℃ for 16 hrs. After cooling, 2 mL of nitric acid (HNO3) and 10 mL of 1 molar hydrochloric acid (HCl) were added. After digestion, samples were filtered using Whatman filter paper and the filtrate is made up to 25 mL with distilled water [7]. To determine the metals (Cr, Cd, Fe, Ni, Cu, Zn, and Pb) in muscles of the fish samples, a GBC (Savant AA Sigma) flame atomic absorption spectrometer (AAS) was used. All chemical reagents were analytical reagent grade (Merck). The glassware and plastic containers were acid washed with nitric acid 10% and rinsed with double distilled water before use and the results expressed as mg/kg dry weight of the fish sample.
The estimated daily intake of each heavy metal was calculated (USEPA, 2015) [8] using the following equation:
Where; EF = Exposure frequency (365 days year-1); ED = Exposure duration (30 years for adults), equivalent to the average lifetime; FIR = Fish ingestion rate (kg person-1 day-1) (19.5 g per person day-1); C = Metal concentration in fish (mg kg-1); RfD = Oral reference dose (mg kg-1 day-1 ); WAB = Average body weight (kg), (60 kg for adults); ATn = Average exposure time for non-carcinogens (365 days year-1 × ED). The exposure duration describes the extent a person may be exposed to a certain pollutant per day/year, while exposure frequency describes the entire period of exposure occurrence in years. The exposure duration and frequency used to characterize non-cancer risk were 365 d/yr for 30 yr, while the averaging time of 365 d/yr for 70 yr was used to characterize lifetime exposure for cancer risk [9]. The fish ingestion rate is the per capita consumption of fish by human, which is equivalent to 19.50 g per day. The reference dose (RfD) and carcinogenic potency slope factor (CPSo) used for health risks (THQ and TR) evaluation are provided by USEPA [9].
The target hazard quotient (THQ) is an estimate of the non-carcinogenic risk level due to metal exposure. To estimate the human health risk from consuming metal contaminated fish, the target hazard quotient (THQ) was calculated [8] by the following equations:
The oral reference dose (RfD) for Ni = 0.05, Zn = 0.3, Cd = 0.001, Pb = 0.004, Cr = 0.003, Cu = 0.04, Fe = 0.7 µg g-1 day-1 [9].
The hazard index (HI) is expressed as the sum of the target hazard quotients (THQ) [9] and calculated by following the equations:
Target cancer risk (TR) was used to estimate the carcinogenic risks. The model for estimating TR [9] is shown as follows:
Where, TR is the target cancer risk and ATc is the averaging time, carcinogens (365 days/year for 70 years). The cancer slope factors for ingested nickel = 1.7, Pb = 0.009 and Cd = 0.6 [9].
The data were statistically analyzed using the statistical package, SPSS 22.0 (SPSS, USA). The means and standard error of the metal concentrations in fish species were analyzed. Multivariate post hoc Duncan multiple range tests and Pearson correlation coefficient were employed to examine the significant differences at p < 0.05 for each metal across different seasons and lagoons.
The mean concentration of heavy metals in C. nigrodigitatus from Ologe and Badagry lagoons are presented in Table 1. There were considerable variations observed in the concentration of heavy metals among different lagoons. This variation may be due to the difference in the accumulation capacities of the species with the anthropogenic activities across each sampling site. The ANOVA of the metals exhibited significant differences (p < 0.05) in Cr, Fe, Ni in the muscle of C. nigrodigitatus from Ologe lagoon. Although the metal concentrations in the muscles of C. nigrodigitatus were below the permissible level of FEPA/WHO standards, the level of the metals was significantly higher in the Ologe lagoon compared to Badagry lagoon across both seasons.
Table 1: Heavy metal concentrations (mg/kg) in C. nigrodigitatus from Ologe and Badagry lagoons. View Table 1
Cluster analysis was performed to depict a dendrogram with a paired group (UPGMA) and Bray-Curtis coefficient of similarities in Figure 2. Cluster analysis was used to provide grouping with similar environmental variables (metals) emerging from the same source. The CA of metals in the muscles of C. nigrodigitatus from Ologe lagoon showed 2 groups: 1) Group 1: 1A - Ni and Cr; 1B - Cd and 1C - Cu and Pb; 2) Group 2 had Fe and Zn as presented in Figure 2a. While at Badagry lagoon, the CA also exhibited 2 groups with (1) Group 1 had 1A - Cu and Pb; 1B - Cd and Ni; 1C - Cr and group 2 had Fe and Zn as presented in Figure 2b. These groups indicate the metals are from similar source anthropogenic discharges into Ologe and Badagry lagoons respectively.
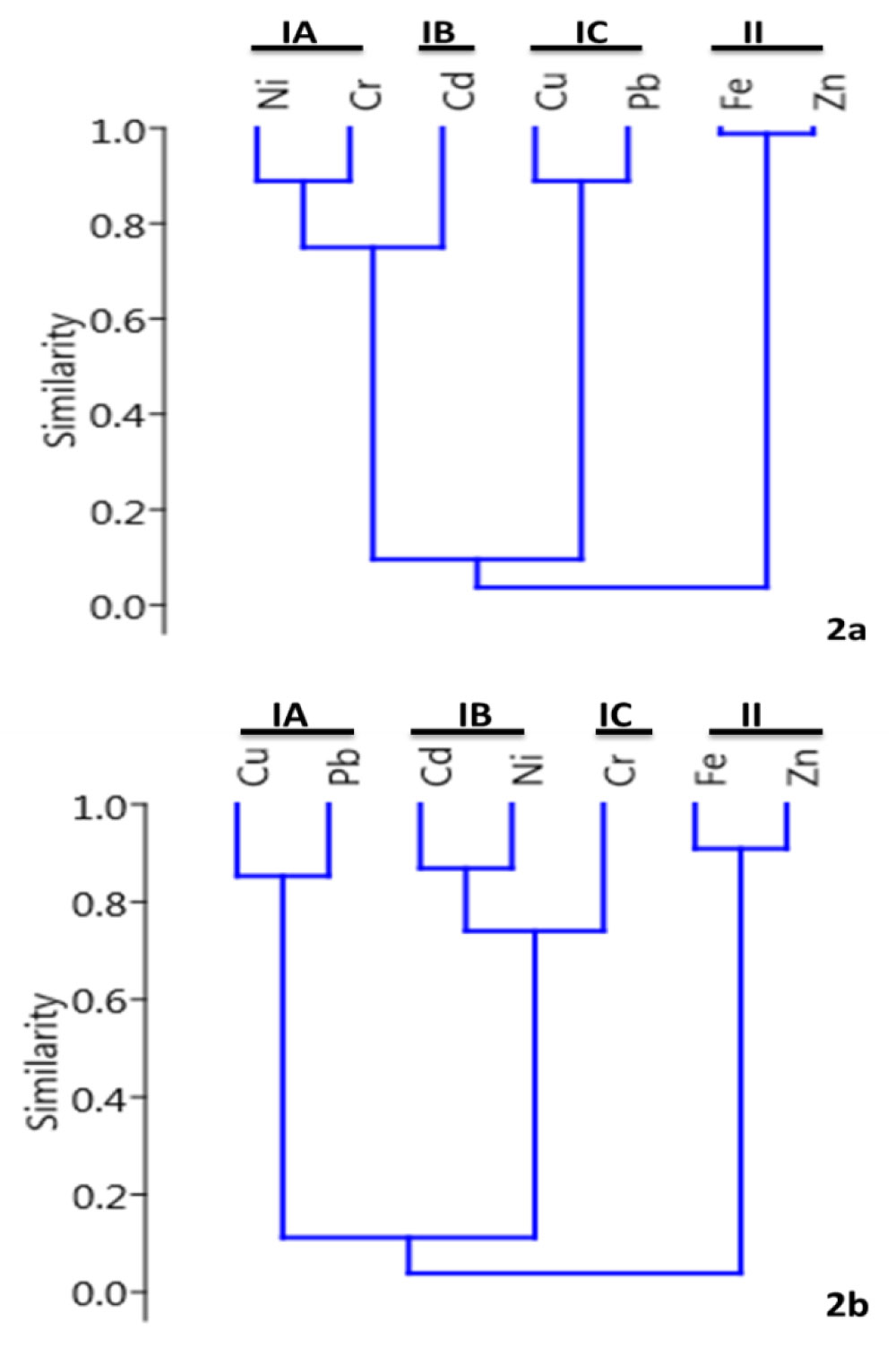 Figure 2: Dendrogram showing cluster of metals based on similarity in (a) Ologe and (b) Badagry lagoons.
View Figure 2
Figure 2: Dendrogram showing cluster of metals based on similarity in (a) Ologe and (b) Badagry lagoons.
View Figure 2
The Pearson correlation coefficient of heavy metals in muscle of C. nigrodigitatus as presented in Table 2, showed that Cu and Pb (r = 0.990*, p < 0.05), Cr and Cu (r = 0.962*, p < 0.05), Cr and Pb (r = 0.985*, p < 0.05) exhibited positively significant correlation. The positive correlation between the metals could be attributed to discharges from non-point sources of heavy metals input into the lagoons.
Table 2: Pearson Correlation Coefficient of metals in C. nigrodigitatus. View Table 2
The health risks resulting from the consumption of Chrysichthys nigrodigitatus purchased from Ologe and Badagry lagoons have been estimated based on daily intake (EDI) and target hazard quotient (THQ) as presented in Table 3 and target cancer risk (Table 4).
Table 3: Estimated daily intake and target hazard quotients (THQ) for individual metals from fish consumption in Ologe and Badagry lagoons. View Table 3
Table 4: Estimated Target Cancer Risk (TR) for individual metals from fish consumption in Ologe and Badagry lagoons.View Table 4
The target hazard quotient (THQ) of the pollutants decreased in the following order: Pb > Zn > Cd > Fe > Cu > Cr > Ni and the hazard index (HI) risk values were 0.204 and 0.202 during wet and dry season at Ologe lagoon; 0.113 and 0.167 during wet and dry season at Badagry lagoon respectively. The THQ for all heavy metals estimated across the seasons and lagoons were less than 1. The contribution of individual THQ values to the HI was evaluated and the results showed that Cd, Zn, and Pb contributed over 80.19% (at Ologe lagoon) and 83.46% (Badagry lagoon) to the combined THQ through this primary exposure pathway of edible muscle (Table 5). Therefore, for the non-carcinogenic risks, more attention should be paid to Cd, Zn and Pb pollution in the study area. The target cancer risk (TR) observed the highest value of 4.3 × 10-5for Ni at dry season and lowest value of 4.0 × 10-6 for Pb in both seasons at Ologe lagoon; while at Badagry lagoon the highest value had 5.20 × 10-5for Ni at the dry season and lowest value of 2.0 × 10-6 for Pb during the wet season respectively.
Table 5: Mean percentage contribution of Target Hazard Quotient (THQ). View Table 5
Measuring the levels of metals in aquatic biotamay serve as a bioindicator of environmental impact on organism and ecosystems health status in comparison with statutory guidelines [10]. The concentration of heavy metals was assessed in the muscles of C. nigrodigitatus and the associated potential health risk in consuming fish from Ologe and Badagry lagoons in southwestern Nigeria. The heavy metal concentrations in the fish species across the study areas were generally below recommended WHO/FMEnv standards. This would imply that the accumulations of these metals in the tissues of C. nigrodigitatus are gradual, although the bioavailability of the metals is attributed to continuous discharges of pollutants from several anthropogenic activities within the study area.
Activities from industrial processes such as metal coating and smelting as well as fertilizers are significant sources of cadmium within Ologe coastal ecosystems. The concentrations of Cd in the muscles of C. nigrodigitatus in this study were below the recommended WHO/FMEnv limit of 2 mg/kg. The continuous accumulation of Cd in fishery resources and biomagnified along the food chain may result in kidney failure and prostate cancer in humans [11,12].
Pb is mostly ubiquitous as a pollutant in water and sediment. The presence of Pb in these study areas are generated from wastewater discharges from local textile factories, printing press cartridges waste and other industrial effluents. The fish species (C. nigrodigitatus) is a demersal species that burrows at the bottom to scavenge for food bioconcentrated with Pb. The levels of Pb in the fish species from both lagoons were below the recommended WHO/FMEnv limit of 2 mg/kg. Pb has tendencies to induce serious threats to the health status of humans. El-Ghasham, et al. [13] stated that Pb is capable of inducing oxidative damage to some organs in the human. The mechanism for Pb-induced oxidative stress even at low concentrations in any aquatic environment includes effects on membranes, DNA, as well as antioxidant defense systems of cells [14]. This study showed that the levels of Pb might be low but has the potential to induce several diseases such as hypertension, kidney disease, neurodegenerative disease, and cognitive impairment in humans from ingesting fish species exposed to lead over a period of time. Zn and Cu are essential elements required for certain biological functions such as growth and metabolism in humans [15]. The concentrations of Zn and Cu in the muscles of C. nigrodigitatus across both lagoons were below the recommended WHO/FMEnv limit of 75 mg/kg and 1 mg/kg.
The EDI values obtained in this study were generally higher compared to the findings of Moslen M and Miebaka [16] recorded EDI values of 0.85 × 10-3 for Pb, 0.45 × 10-3 for Cr, 0.93 × 10-3 for Cu and 0.063 × 10-3 for Cd in Sarotherodon melanotheron for the Health Risk Assessment of Consumption of Fish from an Estuarine Creek in the Niger Delta Nigeria and Abubakar, et al. [17] also had EDI values of 1.162 × 10-3 for Cd, 5.83 × 10-3 for Pb, and 3.91 × 10-3 for Ni from imported frozen Scomber scombrus species sold in Nigeria. The target hazard quotient (THQ) of the metals in this study showed had individual metal values below 1, which suggests a minimal risk of non-carcinogenic consequence per metal.
The Hazard Index (HI) of the summed metals could have synergistic effects in humans. The HI values in this study had values of 0.20 and 0.14 which are less than 1 (HI < 1) in Ologe and Badagry respectively and thus implies minimal non-carcinogenic risk exposure with no significant health risk to consumers of Chrysichthys nigrodigitatus. Studies of Islam, et al. [18] and Zodape [19] also shown that HI values greater than 1 should trigger public health concern for consumers of fishery products. The target carcinogenic risk (TR) estimated in this study observed that all metals were within the guideline values, which implies that fish species from Ologe and Badagry lagoons are safe for human consumption, although may have the probability of contracting cancer due to Pb and Ni exposure over a long lifetime of 70 years or more in future.
In this study, the heavy metal concentrations in C. nigrodigitatus were generally below the WHO/FMEnv guideline values. The estimation of non-carcinogenic risk (THQs and HI) conducted in this study indicated no adverse health effects from the consumption of fish. However, target cancer risk (TR) due to Pb and Ni exposure through fish consumption may increase the probability of developing cancer in the future. The assessment of potential health risk and metal accumulation in the muscle of Chrysichthys nigrodigitatus underscores the need for periodic biomonitoring of the coastal environments. However, the health management implementation commences with regulatory agencies from routine monitoring of facility storage of effluents, toxicological analysed treated effluents following standard methodologies and public awareness on the health implications. The data obtained from this study also show that C. nigrodigitatus possess the requisite feature for use as a bio-indicator for metal pollution monitoring.