Few data are available for excipients administered by inhalation route. This study evaluated the in vitro potential toxicity of three surfactants (Polysorbate 20, Polysorbate 80 and Poloxamer 188) by using an original air-liquid interface (ALI) method of exposure compared to liquid/liquid (L/L) model. Two cell toxicity tests were conducted on BEAS-2B cells, a human immortalized bronchial epithelial cell lines; measurement of Lactate Dehydrogenase activity and XTT cell proliferation assay. We found that Polysorbate 20 appeared to be more toxic than Polysorbate 80, Poloxamer 188. An increased toxicity of Polysorbate 20 in L/L system as shown in comparison to ALI exposure. A toxicity was also observed for polysorbate 80 but at higher concentrations and without difference between L/L and ALI exposure. No toxicity was observed for Poloxamer 188 at high concentrations.
Poloxamer 188 seems to be the better candidate, out of the three tested, for galenic formulations designed to the inhalation route such as biotherapies. To evaluate the cytotoxicity of excipients for inhalation route the ALI exposure have to be used instead of L/L.
Excipients, Surfactants, Toxicity, ALI exposure, Inhalation route
The toxicity of excipients is often considered as secondary to that of active ingredients. Indeed, excipients can be an underestimated source of intrinsic toxicity which may vary depending on the dosage and the route of administration. currently, the use of excipients is based on historical data. However, there are recommendations for industrial use made by the Food and Drug Administration (FDA), the European Medicines Agency (EMA) and the International Pharmaceutical Excipients Council (IPEC) [1-6]. Even with these recommendations, the risk assessment for some excipients appears to be incomplete. Surfactants, such as Polysorbates and benzalkonium chloride are commonly used in galenic formulations for the inhalation route, but data on their toxicity by inhalation pathway are not available.
Furthermore, thanks to its pharmacological characteristics, the inhalation route could provide a solution for the administration of new biotherapies, such as monoclonal antibodies, which are being developed more and more today [7-9]. Taking into account the specificity of the inhalation route, we evaluated the in vitro potential toxicity of excipients by using an original air-liquid interface (ALI) method of exposure.
Three surfactants were selected; Polysorbates 20 and 80 which are already used in formulations for the inhalation route and Poloxamer 188 as a potential candidate for the inhalation route [7-9]. All surfactants were supplied by Sigma-Aldrich (Saint-Quentin Fallavier, France).
Polysorbate 20 (Tween® 20), Polysorbate 80 (Tween® 80) and 10% Poloxamer 188 were sterile-filtered. A 10% stock solution volume (v/v) in PBS was produced for each surfactant except for the 10% Poloxamer 188 which was available as a solution. They were then sterilized through a 0.22 µm filter. The lower dilutions, used to carry out exposures, were obtained from the stock solution by dilution in the culture medium. The concentrations used are detailed below.
BEAS-2B cell lines, supplied by ATCC (Manassas, USA), which are human bronchial epithelial cells immortalized via transfection of the T antigen, were used. This cell line is a good model for aerosol toxicology study [10].
Two exposure models were compared: A classical Liquid/Liquid (L/L) model and an ALI model to approximate the inhalation route exposure conditions and to compare the two models.
L/L exposures were performed on 6-well plates (Corning Inc., New York, USA) preliminary treated with fibronectin at 0.01 mg/ml and inoculated with 600,000 cells in 2 ml per well. For both LDH and XTT test, the Polysorbate 20 concentrations tested were: 0.6%, 1%, 2%; for Polysorbate 80: 1%, 2%, 4% and for Poloxamer 188: 5%, 10%.
ALI exposures were carried out on 6-well plates with inserts (Corning Inc., New York, USA). The inserts were pre-treated with fibronectin at 0.02 mg/ml, inoculated with 400,000 cells per insert in 1.5 ml of culture media and 2 ml of culture media were added to each well. 24 hours after seeding, when the cells were at confluence, they were exposed to surfactants with a nasal spray system (AptarPharma, Le Neubourg, France). Exposures were carried out by spraying 50 µl of the surfactants tested, at a distance of 3.2 cm (optimal measuring distance to cover the surface of the insert). For both LDH and XTT test, the Polysorbate 20 concentrations tested were: 0.6%, 1%, 2%; for Polysorbate 80: 1%, 2%, 4% and for Poloxamer 188: 5%, 10%. In order to get as close as possible to in vivo inhalation exposure conditions, only one exposure was made on the cells, the surfactants were kept 15 minutes and the exposure was stop by cleaning up the cells with the medium. The results were compared to their control values and are the average of at least 3 independent experiments.
In order to determine the toxicity of those chemicals, two cell toxicity tests were conducted, i.e. the measurement of lactate dehydrogenase (LDH) activity and XTT cell proliferation assay.
The LDH test was made by using a kit according to manufacturer instructions (Sigma- Aldrich, Saint-Quentin Fallavier, France).
The adherent cells were exposed to a solution of XTT at 1 mg/ml (Sigma-Aldrich, Saint-Quentin Fallavier, France) with PhenazineMethosulphate at 7.56 µg/ml (Sigma -Aldrich, Saint-Quentin Fallavier, France) for 4 h at 37 ℃. Absorption of the orange formazan formed was measured by spectrophotometry at 450-650 nm.
Statistical analysis was performed with IBM®-SPSS® Statistics 22.0 software (IBM Corp. 2013. IBM SPSS Statistics for Windows, Version 22.0 Armonk). Statistical tests were performed by Mann Whitney tests were done on data because of the small sample sizes. All p-values were two-sided and p < 0.05 was considered as statistically significant.
There is a significant increase in LDH release on some tested concentrations for Polysorbate 20 and Polysorbate 80. For L/L exposure, with Polysorbate 20, 39.2% of LDH increase release was reached after an exposure at 0.06% concentration, 176.7% at 1% concentration and 247.3% at 2% concentration. For ALI exposure, with Polysorbate 20, no significant LDH increase release was reached after an exposure at 0.06% concentration, 85% at 1 v/v% concentration and 166.1% at 2% concentration. For L/L exposure, with Polysorbate 80, no significant LDH increase release was reached after an exposure at 1 v/v% and 2 v/v% concentrations, and 122.4% at 4 v/v% concentration. For ALI exposure, with Polysorbate 80, no significant LDH increase release was reached after an exposure at 1 v/v% and 2 v/v% concentrations and 117.3% at 4 v/v% concentration. For L/L and ALI exposure, with Poloxamer 188, no significant LDH increase release was reached at 5 v/v% and 10 v/v% (Figure 1). The toxicity of Polysorbate 20 appears to be higher than Polysorbate 80 whereas the toxicity of Poloxamer was lowest in both the condition we L/L and ALI exposures at high concentrations (5 and 10 v/v%).
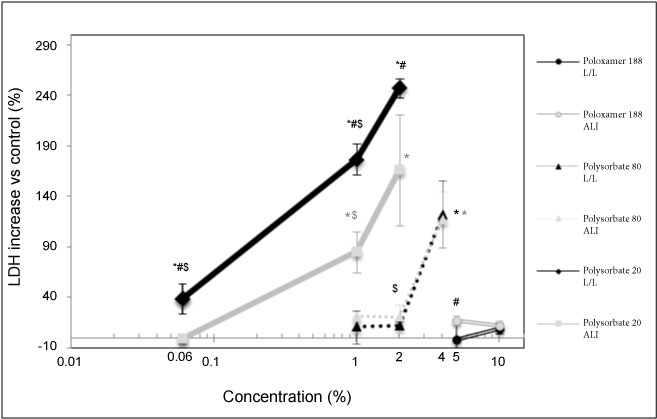 Figure 1: Release of LDH, reported to the negative control, measured in cellular media as a function of surfactant concentration. Significant differences all measurements composing the point vs. control are indicated where *p < 0.05. Significant differences ALI vs. LL are indicated where #p < 0.05. Significant differences vs. the higher concentrations in the same conditions are indicated where $p < 0.01.
View Figure 1
Figure 1: Release of LDH, reported to the negative control, measured in cellular media as a function of surfactant concentration. Significant differences all measurements composing the point vs. control are indicated where *p < 0.05. Significant differences ALI vs. LL are indicated where #p < 0.05. Significant differences vs. the higher concentrations in the same conditions are indicated where $p < 0.01.
View Figure 1
The results of the experiment show a higher toxicity of L/L versus ALI exposures for Polysorbate 20 but not for Polysorbate 80.
The results of the XTT test yield conclusions that are fairly close to the LDH test.
For L/L exposure, with Polysorbate 20, we found 85.2% of living cells, vs. control, after an exposure at 0.06% concentration, 16.6% at 1% concentration and 0.7% at 2% concentration. For ALI exposure, with Polysorbate 20, we found 92.8% of living cells, vs. control after an exposure at 0.06% concentration, 80.1% at 1 v/v% concentration and 77.5% at 2% concentration. For L/L exposure, with Polysorbate 80, we found 76.6% of living cells, vs. control, after an exposure at 1 v/v% concentration, 73% at 2 v/v% concentration, and 33.6% at 4 v/v% concentration. For ALI exposure, with Polysorbate 80, we found 92.6% of living cells, vs. control, after an exposure at 1 v/v% concentration, 87.7% at 2 v/v% concentration and 66.3% at 4 v/v% concentration. As observed with LDH assay, the BEAS-2B cells exposure to Poloxamer 188 revealed a less or no cytotoxicity even at high concentrations, from 5 v/v% to 10 v/v%. There is a higher toxicity of Polysorbate 20 versus Polysorbate 80. The results also showed an increased toxicity of L/L vs. ALI exposure for Polysorbate 20 but not for the Polysorbate 80 (Figure 2).
 Figure 2: Cellular viability, relative to negative control, as a function of the surfactant concentration measured by the XTT assay. Significant differences of all measurements composing the point vs. control are indicated where *p < 0.05. Significant differences ALI vs. LL are indicated where #p < 0.01. Significant differences vs. the higher concentrations in the same conditions are indicated where $p < 0.01 and €p < 0.05.
View Figure 2
Figure 2: Cellular viability, relative to negative control, as a function of the surfactant concentration measured by the XTT assay. Significant differences of all measurements composing the point vs. control are indicated where *p < 0.05. Significant differences ALI vs. LL are indicated where #p < 0.01. Significant differences vs. the higher concentrations in the same conditions are indicated where $p < 0.01 and €p < 0.05.
View Figure 2
During this preliminary study, we focused on the toxicity of surfactants in a BEAS-2B bronchial epithelial cell model, in order to assess a cytotoxic activity of these surfactants and to be able to determine the maximum threshold concentration acceptable in a galenic formulation for the inhalation route that will be tested in future experiments.
We used the innovative ALI exposure in comparison to classical immerged models. In accordance with recent results, less toxicity was found with ALI [11]. However, a significant toxicity of the two Polysorbates remains when using this more realistic ALI exposure. LDH is an intracellular enzyme present in large amount in the cytoplasm, it's know that a loss of membrane integrity will cause a release of LDH into the extracellular medium [12]. Our results suggest that Polysorbates 20 and 80 induce damage the cell membrane integrity. Further, XTT is a tetrazolium salt which allows the measurement of mitochondrial dehydrogenase activity demonstrating cell viability [13]. Our results highlight that Polysorbate 20 and to a lesser extent, Polysorbate 80 impairs mitochondrial function. The toxic concentrations found for these surfactants were high (1 to 2 v/v%).
This should be kept in perspective, especially referring to the maximum usable concentrations recommended by the FDA for inhalation, available only for Polysorbate 80 at the concentration of 0.02% for which no toxicity has been shown but on a single exposure. Since the concentrations used by manufacturers in their galenic formulations are not available, it is difficult to obtain a point of comparison for the toxicity of these excipients. But we know that the excipients appear in weight order in the formulations and when we look, for example, at the composition of the inhaled Beclometasone, Polysorbate 20 appears to be the excipient present in the greatest quantity. We can therefore ask whether manufacturers are complying with the maximum concentrations recommended by the FDA. The results presented here are mainly focused on single exposures for very short duration, however, the use of Polysorbate 20 and 80 for inhalation routes should also be evaluated in long-term repeated exposure. In addition, the half-life of surfactants by inhalation is unknown and it is possible that the exposure time may be much longer than the exposure times tested in this study.
Poloxamer 188 seems to be the safest surfactant among those tested. Indeed, it showed no significant toxicity on all the tests done at high concentrations (5 and 10 v/v%) for ALI and L/L exposures. We originally showed the cytotoxicity of surfactants, Polysorbate 20 and 80 on human bronchial epithelial cells using 2 endpoints of cytotoxicity and two methods of exposure (ALI and L/L). This will complete the few data existing for such excipients in order to select the best excipient candidates for the inhalation route at the relevant free-toxicity concentration. Considering this preliminary result, Poloxamer 188 could be a better choice of excipient in an inhaled formulation than the Polysorbate currently in use.