Food industries are among the major contributors to industrial wastes. Their wastewaters can pose a threat if untreated and indiscriminately disposed into the environment. In this study, we investigated the potential genetic and reproductive toxicity of Cocoa processing industry wastewater, by using the mouse Micronucleus (MN) and sperm morphology assays, respectively. Some of the physico-chemical properties of the wastewater were also determined. The bioassays were carried out at concentrations of 1, 5, 10, 25 and 50% of the wastewater. Assessment of sperm shape showed that the fraction of the sperm that was abnormal in shape was significantly (p < 0.05) greater than the negative control value. There was also a significant (p < 0.05) reduction in the mean sperm count of the exposed mice compared to the control. MN analysis showed a concentration-dependent induction of micronucleated polychromatic erythrocytes across the treatment groups. These observations were provoked by the toxic and genotoxic constituents present in test sample. The tested Cocoa processing wastewater is a potentially genotoxic agent and germ cell mutagen, and may induce adverse health effects in exposed individuals.
Genotoxicity, Cocoa processing industry, Mouse, Spermatozoa, Micronucleus
Cocoa beans, the fruit seeds from the tropical tree Theobroma cacao are highly prized, as the solids and fat provide the basis for cocoa powder and chocolate production. Following bean fermentation and drying in the countries of origin, cocoa beans are transported to industrial plants, where semi-manufactured or finished products are obtained for commercialization [1]. Cocoa products are regarded to be among some of the most widely consumed foods worldwide. Cocoa beans are the raw materials from which the widely patronized products such as chocolate, candies, cocoa powder and beverages are produced. Cocoa processing industries majorly process cocoa beans into four food grade usable products: Cocoa Liquor, Cocoa Butter, Cocoa Cake and Cocoa Powder. Generally, manufacturing processes differ slightly due to the different species of cocoa trees, but most factories use similar machines to break down the cocoa beans into cocoa butter and chocolate [2]. The manufacturing process of making chocolate consumes industry water in several steps including harvesting, cleaning, fermentation, drying, roasting, grinding, pressing, pulverizing and mixing. Liquid raw materials and wastewater produced during the confectionery manufacturing process are likely to have a high organic content, particularly sugars and vegetable fats. Should effluent containing these materials be discharged untreated into water courses it can cause pollution. Most industrial wastewater can be characterized as extremely complex mixtures containing numerous inorganic as well as organic compounds [3]. Industrial wastewater contains offensive and potentially dangerous substances which cause pollution and contamination of the environment and receiving water bodies [4].
Industrial wastewaters are the main source of direct and often continuous input of pollutants or toxicants into aquatic ecosystems with long-term implications on ecosystem functioning [5-7]. It has been reported that industrial wastewater has hazard effect on water quality, habitat quality and complex effects on flowing waters [8]. Industrial wastes and emission contain toxic and hazardous substances, most of which are detrimental to human health [9,10]. Many industrial organic substances found in water can cause death or reproductive failure in fish, shellfish and wildlife and they can accumulate in animal and fish tissues absorbed in sediment or find their way into drinking water supplies, posing long-term health risks to human.
Among the contaminants present in industrial wastewater are heavy metals which are of serious environmental concern in recent years; this is because of their toxicity, bioaccumulation and bioconcentration in living organisms. Also, their persistence in the environment and non-biodegradable nature has heightened its concern [11]. Some of the substances found in wastewaters are genotoxic and are suspected to be a possible cause of the cancers observed in the last decades. Wastewater genotoxicity studies are of interest because epidemiologic investigations have shown a link between genotoxic drinking water intake and a rise in cancers [12]. Wastewater may be mutagenic or toxic and lead to several human afflictions like cancer, cardiovascular diseases and premature ageing [13]. The complexity of the industrial wastewater makes it very difficult to carry out hazard assessment using chemical analysis alone. Therefore, there is need to subject wastewater to various genotoxicity tests before discarding them into the environment.
Many mutagenicity and genotoxicity tests have been used in combination with physical and chemical analysis in order to evaluate water quality [14-20]. The growing interest in these tests is due to the fact that despite the existence of different toxicity mechanisms for various organisms of different species, a substance that is toxic for an organism often demonstrates similar toxic effects on other organisms [21].
In the present study, two eukaryotic assays: Micronucleus (MN) and abnormal sperm morphology assays in mice were used. The physico-chemical and heavy metal analyses of the wastewater were also carried out.
Wastewater was collected from a Cocoa processing factory, at the point of release into the environment, in Akure South Local Government (Latitude 7°28'N and Longitude 5°16'E), Nigeria. The wastewater was collected into a sterile 10-litres plastic container and kept at 4 ℃ throughout the period of this study. Five concentrations: 1, 5, 10, 25 and 50% (v/v, wastewater/distilled water) of the sample were utilized in this study, while distilled water and cyclophosphamide (20 mg/kg body weight) were used as negative and positive controls, respectively.
The wastewater was analyzed for a number of standard physico-chemical properties, including Chemical Oxygen Demand (COD), Total Dissolved Solids (TDS), alkalinity, Biochemical Oxygen Demand (BOD), acidity, chlorides, nitrates, ammonia and phosphates, sulphate according to methods described by APHA [22]. Heavy metals namely Lead (Pb), Cadmium (Cd), Copper (Cu), Chromium (Cr), Iron (Fe), Zinc (Zn), Nickel (Ni) and Manganese (Mn) were analyzed in the wastewater sample according to standard analytical methods [22,23]. Briefly, 100 mL of the wastewater was digested by heating with concentrated HNO3, and the volume reduced to 3-5 mL. This volume was made up to 10 mL with 0.1 N HNO3. Concentrations of the metals were estimated by using an Atomic Absorption Spectrophotometer.
Young male Swiss albino mice (Mus musculus) of 6 and 12-weeks-old, for micronucleus and sperm morphology, respectively, which had been inbred for several generations, were obtained from the animal breeding unit of the Department of Physiology, University of Ibadan, Nigeria. They were kept in a pathogen free, well ventilated animal house at the Department of Biology, Federal University of Technology, Akure, for 2 weeks in order to acclimatize. They were maintained in the same room throughout the period of this study. Mice chow (Ladokun pelleted feed®) and drinking water were supplied ad libitum.
Induction of sperm abnormalities was studied according to Wyrobek, et al. [24] and Bakare, et al. [25]. Concentrations of 1, 5, 10, 25 and 50% (v/v, wastewater/distilled water) of the Cocoa industry wastewater were considered together with the positive (cyclophosphamide 20 mg/kg bodyweight) and negative (distilled water) controls. A single Intraperitoneal (IP) injection of 0.5 ml of the different test-sample concentrations was administered to the mice in different cages daily for 5 consecutive days. The IP route was favored since it is one of the fastest and most efficient means of delivering test-chemicals into test-animals in a short-term-assay. Sperm was obtained from the caudal epididymes after five weeks from the first injection, since spermatogenesis in mice takes 34.5 days until completion [26]. The mice were sacrificed by cervical dislocation and their epididymes surgically removed. Two sperm suspensions were prepared from the caudal of each testis by mincing the caudal in physiological saline. Smears were prepared on grease-free slides after staining with 1% Eosin Y for 45 min. The slides were air-dried and coded for subsequent microscopic examination under oil immersion at 1000×. For each mouse, 1000 sperm cells were assessed for morphological abnormalities according to the criteria of Wyrobek and Bruce [27].
The caput epididymes in the testes of each mouse in the sperm morphology assay were surgically removed and minced in physiological saline. The counting of sperms was made from their suspension with the aid of counting chamber of Neubauers' hematocytometer at 400× [28]. Pooled sperm count from the mice in each group was expressed as mean sperm count per milliliter of suspension.
Whole blood smears were collected on the day following the last wastewater administration. Whole blood smears were prepared on clean microscope slides, air dried, fixed in methanol and stained with acridine orange (125 mg/ml in pH 6.8 phosphate buffer) for 1 min just before the evaluation with a fluorescence microscope using a ×40 objective [29]. The frequency of Polychromatic Erythrocytes (PCEs) per total erythrocytes was determined using a sample size of 2000 erythrocytes per animal. The number of Micronucleated Polychromatic Erythrocytes (MNPCEs) was determined using 2000 PCE per animal. Briefly, immature erythrocytes (i.e. PCEs) were identified by their orange-red color, mature erythrocytes by their green color and micronuclei by their yellowish color.
Data were compared by one-way variance analysis. Statistical analysis was performed using SPSS for Windows 9.05 package program. Multiple comparisons were performed by least significant difference test. P < 0.05 was considered to be the level of significance.
Physical and chemical characteristics of the Cocoa processing industry wastewater are shown in Table 1. Chloride and nitrate levels were low, however, the levels of Fe, Cu, Pb, Cr, Cd, Mn and Ni were higher than maximum allowable limit by standard organizations [23,30].
Table 1: Physico-chemical and heavy metals analysis of Cocoa processing industry wastewater. View Table 1
The results obtained in the sperm morphology assay are presented in Table 2. Cocoa processing industry wastewater induced different types of sperm abnormalities such as "hookless", "banana", "short hook", "nubbed hook". "double tail", "hook at wrong angle", "wrong tail attachment", "amorphous", and "folded" sperms at all concentrations tested (Figure 1). The increase in abnormal sperm cells were concentration-dependent and statistically significant (p < 0.05) from 10% to 50% concentrations. "Amorphous head" was the most common abnormality observed in the sperm cells of exposed mice (Figure 2).
Table 2: Summary of Morphologically Abnormal Sperm Cells and mean (mL) sperm count induced in mice after five weeks' exposure to different concentrations of Cocoa Processing Industry wastewater. View Table 2
 Figure 1: Abnormal sperm cells induced in mice exposed to different concentrations of Cocoa processing Industry wastewater A) Normal sperm cell; B) Hook at wrong angle; C) Wrong tail attachment; D) Amorphous head with extention; E) Amourphous head; F) Short hook; G) Wrong tail attachment and amorphous head; H) Knuubed hook (×1000). View Figure 1
Figure 1: Abnormal sperm cells induced in mice exposed to different concentrations of Cocoa processing Industry wastewater A) Normal sperm cell; B) Hook at wrong angle; C) Wrong tail attachment; D) Amorphous head with extention; E) Amourphous head; F) Short hook; G) Wrong tail attachment and amorphous head; H) Knuubed hook (×1000). View Figure 1
 Figure 2: Percentage frequencies of the different types of sperm abnormalities observed in mice treated with different concentrations of Cocoa processing industry wastewater. View Figure 2
Figure 2: Percentage frequencies of the different types of sperm abnormalities observed in mice treated with different concentrations of Cocoa processing industry wastewater. View Figure 2
Table 2 further shows the result of the mean sperm count in the exposed mice. The negative and positive controls had 5.02 × 106 mL-1 and 4.09 × 104 mL-1 mean sperm counts, respectively. The mean sperm count was significantly lower (p < 0.05) at 25 (7.05 × 105 mL-1) and 50 (5.09 × 105 mL-1) % concentrations of the wastewater compared to the negative control.
Figure 3 shows the results of PCE and MNPCE observed in the peripheral blood of mice exposed to Cocoa processing industry wastewater. There was significant (p < 0.05) induction of MN by the wastewater, which is concentration-dependent from 10-50% concentrations compared to the negative control. The mean of MN in the negative control was 5.85 as compared to 6.21, 6.85, 10.21, 12.55, 14.88 and 15.27 for 1, 5, 10, 25, 50% concentrations of the wastewater and the positive control (cyclophosphamide), respectively. Figure 4 shows the MN induced in the peripheral blood of mice exposed to the different concentrations of Cocoa processing industry wastewater.
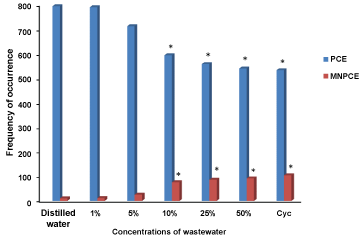 Figure 3: The mean of PCE and MNPCE induced in the peripheral blood of mice exposed to the different concentrations of Cocoa processing industry wastewater. *significant at p < 0.05 compared to the negative control; PCE: Polychromatic Erythrocytes; MNPCE: Micronucleated Polychromatic Erythrocytes; Cyc: Cyclosphosphamide. View Figure 3
Figure 3: The mean of PCE and MNPCE induced in the peripheral blood of mice exposed to the different concentrations of Cocoa processing industry wastewater. *significant at p < 0.05 compared to the negative control; PCE: Polychromatic Erythrocytes; MNPCE: Micronucleated Polychromatic Erythrocytes; Cyc: Cyclosphosphamide. View Figure 3
 Figure 4: Micronucleated polychromatic erythrocytes induced in the peripheral blood of mice exposed to the different concentrations of Cocoa processing industry wastewater under flourescent microscope. View Figure 4
Figure 4: Micronucleated polychromatic erythrocytes induced in the peripheral blood of mice exposed to the different concentrations of Cocoa processing industry wastewater under flourescent microscope. View Figure 4
The present study reports the genetic and reproductive toxicity of Cocoa processing industry wastewater in both somatic and germ cells in mice. The results of this study revealed that the wastewater significantly increased the percentage of abnormal sperm in mouse germ cells from 10% concentrations and above. These findings suggest that Cocoa processing industry wastewater contains agents which might be germ cell mutagens. The exact reason of the increase in the frequency of abnormal sperm was not clear and opinions on this subject differ. The induction of abnormal sperms has been suggested to be a result of an abnormal chromosome [31], minor alteration in testicular DNA [32], and point mutation [33]. According to several studies [34-37], small deletions, point mutations, and abnormal chromosomes were proposed as possible genetic causes of such alterations. Bruce and Heddle [38] attributed the occurrence of sperm head abnormalities to the chromosomal aberrations that occur during the packaging of genetic material in the sperm head or occurrence of point mutation in testicular DNA. Several studies [25,39] reported that abnormalities may also arise as a consequence of mistakes in the spermatozoa-differentiating process during spermatogenesis. Another two studies [40,41] reported that abnormalities in sperm heads may occur by physiological, cytotoxic or genetic mechanisms or alterations in testicular DNA which in turn disrupts the process of differentiation of spermatozoa. Exposure to chemicals could produce hormonal changes causing disturbances in normal spermatogenesis or could cause abnormalities in the seminal fluid leading to structural or functional impairment of the sperms [42]. Decrease in live sperm and increase in the number of abnormal sperm may also be due to enhanced ROS production by the wastewater in the testis and epididymis. Wastewater induced ROS production is known to adversely affect sperm motility, live sperm and increase sperm abnormality [43].
The reproductive toxicity of Cocoa processing industry wastewater was further confirmed by the significant reduction in the mean sperm count of the exposed mice. The results obtained showed that the wastewater does not only contain agents that are germ cell mutagens but also capable of altering the production of sperm cells. Sperm morphology and sperm count have been recognized as predictive tools in assessing a male's reproductive capacity. Reduction in sperm cell production is a major factor causing male infertility. Wyrobek [44] reported that large reductions in sperm number or motility or large increases in sperm with abnormal shapes are associated with reduced fertility.
Previous reports have shown that wastewater from industries contain germ cell mutagens capable of inducing reproductive toxicity in exposed animals. Indeed, induction of abnormal sperm morphology and reduction in sperm count as reported in this study is in accordance with the study of Bakare, et al. [43] where pharmaceutical effluent was reported to induce significant abnormal sperm cells. Alabi, et al. [45] also reported a similar data of tobacco industry wastewater induced abnormal sperm morphology in exposed mice.
The result of the micronucleus assay showed that Cocoa processing industry wastewater induced significant MN in the peripheral blood of exposed mice. The induction of micronuclei in the peripheral blood of mice or any cell or any other organism is the manifestation of chromosome breakage and disturbance of the mitotic process due to spindle abnormalities [13,46]. Micronuclei are considered as an indication of a true mutation effect [47], thus, the high percentage of the micronuclei induced by the studied wastewater sample may indicate the mutagenic effect of it. This study is in agreement with previous studies [48-50], where various industrial wastewaters induced significant, concentration-dependent increase in micronuclei in exposed organisms.
The results of these assays (sperm head abnormality, sperm count and MN) are the indications of reliability and high sensitivity of these bioassays. These results showed that the wastewater contain mutagens. Among those mutagens, heavy metals are a potentially mutagenic class of environmental pollutants and some of them are implicated in the induction of tumors in experimental organisms and exposed humans [51]. It was shown that these metals could induce clastogenic and aneugenic effects including mitosis and cytokinesis disturbances [52]. Cd, Pb, Zn, and Cu have been shown to cause cytogenetic effects mainly from their ability to induce genome damages, enzyme inhibition and react with the tubulin SH group [46,53-55].
The presence of high concentration of heavy metals in the wastewater from Cocoa processing industry as reported in this study is in accordance with the report of Akinnusotu and Aranwade [56], where the levels of Cu, Zn, Mn and Fe in wastewater from three cocoa processing factories were higher than maximum allowable levels.
In conclusion, this study showed that wastewater from Cocoa processing industry contained heavy metals at concentrations higher than maximum allowable limits by regulatory bodies. The constituents of the wastewater were shown to be genotoxic to somatic cells and also capable of inducing reproductive toxicity. These results show that indiscriminate disposal of this wastewater into the environment can pose a threat to organisms in the ecosystem and to humans who are exposed to it.