Introduction: Methods to obtain an immediate and long-term increase in muscle strength and function are goals of rehabilitation practitioners, sports therapists, strength and condition coaches and athletes. There is a growing body of evidence to support the use of acupuncture for rehabilitation of muscle function following stroke and there is strong evidence to support the use of acupuncture to enhance muscle strength in healthy individuals [1]. Acupuncture has been shown to affect both acute and chronic improvement in muscle strength. However, the underlying physiological mechanisms by which acupuncture enhances muscle strength are still unclear and to our knowledge have never been fully investigated. The purpose of the current review is to critically appraise randomized control trials, systematic reviews, and meta-analyses to determine the underlying physiological mechanisms in which acupuncture enhances muscle strength in both animals and humans. This review will lay the foundation for clarifying the likely mechanisms of acupuncture in enhancing acute and chronic muscle strength. Thus, allowing clinicians and researchers involved in sports and/or exercise rehabilitation to justify their selection of acupuncture points.
Conclusion: Acupuncture enhances muscle strength in several ways which include improving the efficiency in which mitochondrial cells function, reducing inflammation, and releasing antioxidants thereby reducing the build- up of reactive oxygen species and damage to cells. Furthermore, acupuncture produces a strong motor-evoked potential. Most studies in this review were undertaken on post-stroke patients; therefore, further studies with a large sample size are required on healthy athletes to help determine the underlying physiological effect of acupuncture on improving muscle strength in sports.
Methods to obtain an immediate and long-term increase in muscle strength and function are goals of rehabilitation practitioners, sports therapists, strength and condition coaches and athletes. Long-term improvement in muscle strength results from ongoing sports-specific strength and conditioning training. There is a growing body of evidence to support the use of acupuncture for rehabilitation of muscle function following stroke and there is strong evidence to support the use of acupuncture to enhance muscle strength in healthy individuals [1]. Acupuncture has been shown to affect both acute and chronic improvement in muscle strength. However, the underlying physiological mechanisms by which acupuncture enhances muscle strength are still unclear and, to our knowledge, have never been fully investigated. The purpose of the current review is to critically appraise randomized control trials, systematic reviews, and meta-analyses to determine the underlying physiological mechanisms in which acupuncture enhances muscle strength in both animals and humans. This review will lay the foundation for clarifying the likely mechanisms of acupuncture in enhancing acute and chronic muscle strength, thus allowing clinicians and researchers involved in sports and/or exercise rehabilitation to justify their selection of acupuncture points. This review is reported according to the SANRA (Scale for the Assessment of Narrative Review Articles) guidelines [2].
There are a several mechanisms by which acupuncture has been suggested to influence muscle strength (see Figure 1). One mechanism is through the activation of regions of the brain following the stimulation of specific classical acupuncture points. The main system in the body that is affected by acupuncture is the nervous system. It not only transmits signals along central, spinal, and peripheral nerves but also emits a variety of biochemicals that influence other cells of the body. The nervous system has over 30 peptides involved in transmitting signals and is connected to the hormonal system via the hypothalamic-pituitary axis. Therefore, it can make connections to every cell and system of the body [3]. In 1973 Chiang and colleagues [4] found that the effect of acupuncture is mediated by the nervous system. This can be explained by the fact that the classical acupoints are situated near underlying nerves. Liu, et al. [5] found a high correlation between acupuncture points and muscle motor points. Dung [6] found that there were, in close vicinity to acupuncture points, large peripheral nerves, motor points where the nerve enters the muscle, blood vessels near neuromuscular attachments, cutaneous nerves emerging from the deep fascia, bifurcation points of peripheral nerves, ligaments, tendons, joint capsules, and fascial sheets, which are rich in nerve endings. The electrical conductivity of acupoints has been known for several decades thanks to the work of Nakatani [7] and Dr Robert Becker [8] in the 1950s, and Saita [9] and Tiller [10] in the 1970s. The electrical properties of acupuncture points include increased conductance, reduced impedance and resistance, increased capacitance, and elevated electrical potential compared with adjacent non- acupuncture points [11].
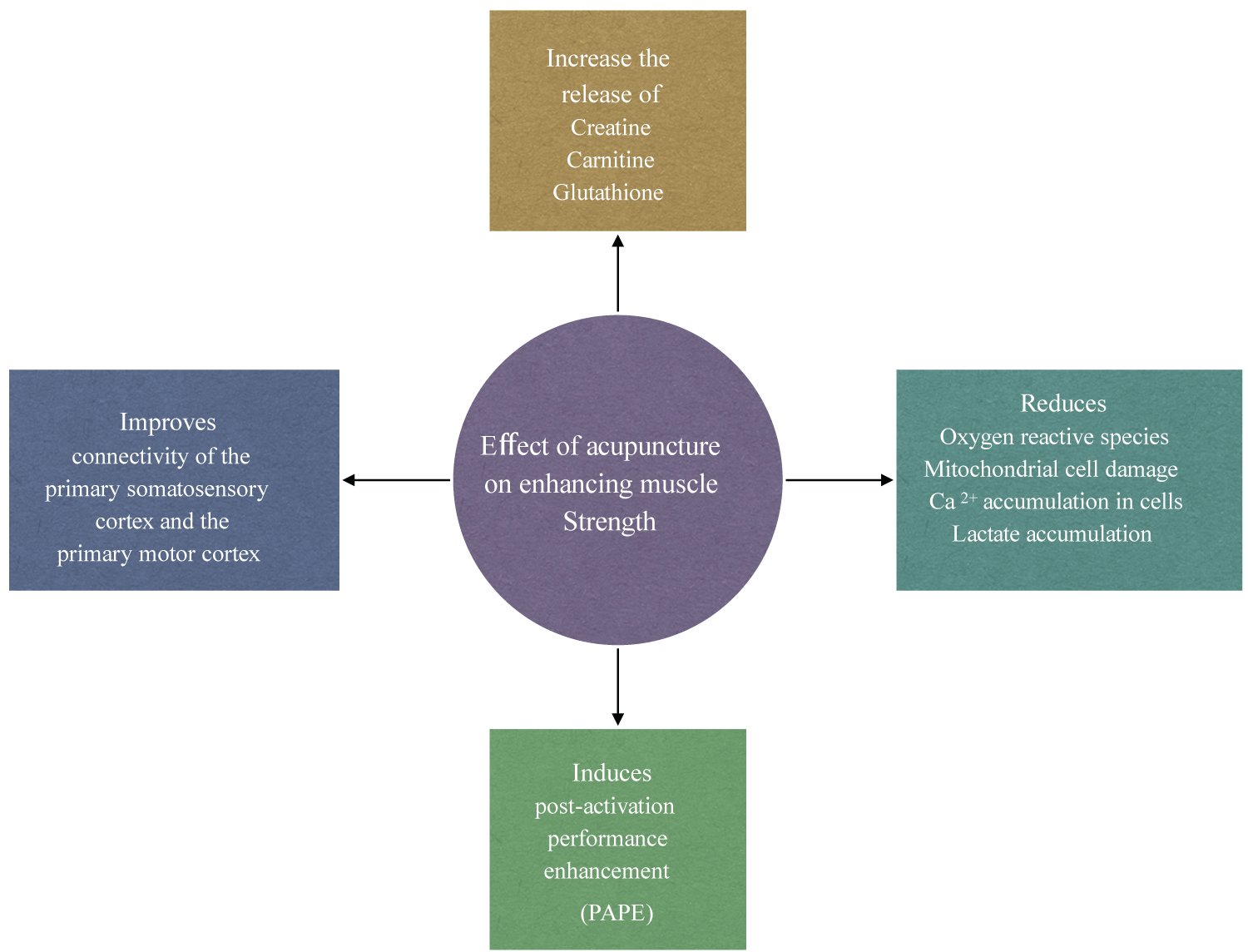 Figure 1: Mechanism of action of acupuncture on improving muscle strength.
View Figure 1
Figure 1: Mechanism of action of acupuncture on improving muscle strength.
View Figure 1
In traditional Chinese medicine (TCM), deqi is one of the most important principles and the key to successful acupuncture treatment since it is associated with clinical efficacy [12-14]. The sensations of deqi are transmitted by nerves local to the needling site to the somatosensory cortex where integration and interpretation occur at the spinal cord, brainstem, thalamic and cortical level [15-18]. Nerves local to the needling site consist of low-threshold nociceptors that are connected to fast-conducting A-delta fibers, which generally supply the skin and respond to strong mechanical stimuli [19]. High-threshold nociceptors that conduct impulses through slower unmyelinated C-fibers carry impulses to deep body tissues such as muscles. The sensation of deqi has been reported to be detected in acupuncture and non-acupuncture points; however, a stronger sensation of deqi is reported at classical acupuncture points in comparison with non-acupuncture points. Kong, et al. [20] found that the sensation of deqi from sham acupuncture was significantly lower than that of Manual Acupuncture (MA) or electroacupuncture (EA). Li, et al. [21] investigated the central mechanism of deqi following acupuncture in the treatment of ischemic stroke and found that, compared with the non-deqi group, the deqi group produced marked activation of the right anterior lobe of the cerebellum and right limbic lobe. These findings are supported by several studies that investigated the efficacy and mechanism of different depths of acupuncture for mild cognitive impairment and found that deep acupuncture is necessary to achieve significant clinical results [22-24]. With regards to the activation of the primary motor cortex, it is suggested that a strong deqi sensation is required. Sun, et al. [25] Wang, et al. [26] and Yang, et al. [27] established that acupuncture at ST-36 acupoint produces a strong deqi sensation that activated different nerve fibers which affect the excitability of the motor cortex and was correlated to Motor-Evoked Potential (MEP). Although there is convincing evidence to support the requirement of a strong deqi sensation at classical acupoints to affect the motor cortex and produced a strong MEP, amplitude studies investigating osteoarthritis have reached conflicting conclusions. Furthermore, Maioli, et al. [28] showed that both classical acupoints and non-acupoints had a similar effect in modulating MEP responses in the motor cortex. Some studies have questioned the need for deep stimulation and deqi in achieving acupuncture analgesia [14,29-33]. Nonetheless, the main findings from the above studies suggest that in order to activate the motor cortex and produce a stronger motor- evoked potential, it would be more beneficial to obtain a strong deqi sensation by applying deep needling (10-20 mm) to classical acupoints.
The acute effect of acupuncture in the enhancement of explosive muscle force may be brought about by stimulating local nerves in the agonist muscle to elicit the deqi sensation. Using acupuncture to stimulate local nerves in the agonist muscle is reported to induce a Post-Activation Potentiation (PAP) phenomenon [34-36], which is a method that is used for strength and conditioning of athletes to improve strength and performance. Data collected during this review indicates that the acute effect of acupuncture on explosive muscle force may be more associated with Post-Activation Performance Enhancement (PAPE) rather than PAP.
The most accepted physiological mechanism that plays a key role in the PAP phenomenon is an increase in the phosphorylation of myosin regulatory light chains [37-39]. This makes actin and myosin more sensitive to calcium released from the sarcoplasmic reticulum during subsequent muscle contractions [40-43]. As a result, there is an increase in the cross-bridge cycling between actin and myosin filaments [44], thereby increasing the force of each subsequent twitch contraction. PAP has been shown to have a short window of action (effect decreases drastically to one-half after 28s) following its induction and is only beneficial in the first 8-10 minutes after being activated [45]. Post-Activation Performance Enhancement (PAPE) is an acute increase in explosive neuromuscular force during high-speed dynamic muscle contractions. It comes on 3-10 minutes following its activation [46,47] and has a longer window of action (> 15 minutes) [48-51] than PAP. The physiological mechanism is reported to be through an increase in neural drive and high-threshold motor unit activation and an increase in muscle temperature and muscle water content. The effect of PAP and PAPE is greater in type II fast-twitch fibers [52] than in type I slow-twitch fibers [53] as type II fibers have a lower basal Ca2+ sensitivity [54,55]. In 2021 Wang and colleagues [26] undertook two studies, one to investigate the immediate effect of acupuncture on the explosive force of the knee joint and the other to investigate the effect of acupuncture on the timeline of male shoulder joint endurance. Both studies demonstrated that selecting and stimulating acupoints around the shoulder joint (LI-14, SJ-14, SJ-13, LU-1, LU-4, LU-3 and SJ-12) or the knee joint (ST-32, ST-34, ST-36, SP-10 and UB-57) activate the nerves and muscles around these joints. Their results showed a significant increase in the maximum torque, average power, average work, and total work of the shoulder and knee joints. Both studies found that acupuncture improved muscle strength for 23-33 minutes following its application. Like PAPE the timeline to the onset of the effect of acupuncture took approximately 10 minutes to appear [56]. Su, et al. [35] compared the acute effect of acupuncture with sham acupuncture on forearm muscle strength. They found that immediately following 20 minutes of acupuncture to LI-4, LI-10, LI-11, SI-8, SI-10, and SJ-5 there was a significant enhancement in forearm muscle force, which lasted approximately 7-20 minutes until the acupuncture efficacy decreased or disappeared. There was no significant enhancement in forearm muscle force in the sham group. De Souza, et al. [57] evaluated the effect of acupuncture on the upper trapezius muscle. They found that following acupuncture there was a significant increase in the upper trapezius muscle strength at 20 minutes following acupuncture and the effects lasted for 5 minutes. The timeline for acupuncture to be effective and the timeline for the efficacy to decrease or disappear coincide with the timeline of the PAPE studies. Therefore, it may be deduced that acupuncture has a similar effect to PAPE on enhancing explosive muscle strength rather than PAP, which has a shorter timeline.
The somatosensory cortex lies behind the primary motor cortex in the frontal lobe. It functions to detect sensory stimuli such as touch, pain, pressure, and movements such as joint displacement [58], which arises from muscles, bones, joints, skin, cardiovascular system, internal organs, and fascia. Sensory information from muscle spindles, musculotendinous receptors concerning changes in muscle length are transmitted to the medial nuclei of the thalamus which decode and process the information before passing this on to the somatosensory cortex [59]. Early motor mapping studies in primates [60] and humans [61] have described movements that are brought about by stimulation of the primary somatosensory cortex in addition to the motor cortex. In support of these findings, there is growing evidence from animal studies showing that rich fiber pathways interconnect the primary somatosensory cortex with the primary motor cortex [62-64]. A study using Transcranial Magnetic Stimulation (TMS) found that somatosensory electrical stimulation strongly increased the excitability of the primary motor cortex [65]. Boricha and colleagues [66] undertook a review to investigate the role of the primary somatosensory cortex in motor control, motor learning and functional recovery. Their findings indicated that without correct processing and translation of sensory input, both before and during movement, motor outputs were abnormal and/or inaccurate. Research on humans has suggested that there is an increased peripheral somatosensory inflow that facilitates functional reorganization of the primary motor cortex [67] and that non-invasive stimulation of the somatosensory cortex induces shorter latencies to initiate movements [68]. These findings support a continuous mutual communication between sensory inflow and motor outflow [69,70]. Non-invasive stimulation of the somatosensory cortex can be brought about through tactile-pressure stimulation as it shares some similarities with non-painful electrical stimuli [25] or acupuncture. Insertion of a needle into the skin or muscle will stimulate free nerve endings, encapsulated cutaneous receptors (Merkel, Meissner receptors, Ruffini, and Pacinian corpuscles), sarcous sensory receptors (muscle spindles and tendon organs), and their afferent fibers [71]. Applying manual manipulation or electrical stimulation to the needle will produce a stronger activation of various neural and neuroactive components thereby producing a strong deqi sensation. This will produce an action potential in C-fibers and A-delta fibers located in the skin and muscle which will travel to the central nervous system, where integration and interpretation occur at the thalamus and somatosensory cortex. This can provide feedback that is required for motor control during exercise rehabilitation or in cases of enhancing sports performance. In healthy humans, it has been found that electrical stimulation over the agonist muscle during exercise results in improved training effects compared with either electrical stimulation or exercise only [72]. Wei, et al. [73] used fMRI to examine the activation and connectivity of the brain of 22 right-handed healthy male volunteers under three different conditions: (1) Right ankle dorsiflexion as the control; (2) Ankle dorsiflexion coupled with tactile-pressure stimulation to the agonist (tibialis anterior) muscle; and (3) To a control area proximal to the right medial malleolus away from the agonist (tibialis anterior) muscle. Tactile stimulation was administered along the tibialis anterior muscle by brushing the skin of subjects in a back-and- forth manner with a probe at a frequency of 1 Hz and a shift distance of 3 cm. They found that increased somatosensory inputs during movement activated links between the primary sensory centers (thalamus and cerebellum) and major motor centers.
In clinical applications, sensory inflow through repetitive stimulation of the peripheral nerves combined with voluntary muscle contractions has been found to enhance motor function in the rehabilitation of post-stroke patients [74-78]. This method of rehabilitation has been shown to induce a more significant muscular adaptation than muscle contraction alone [79,80]. In sports, it has been demonstrated that stimulating the muscles or nerves through an intense exercise- based warm-up will produce a voluntary force or power enhancement for a short time as seen in the PAPE phenomenon. Acupuncture stimulation of the peripheral nerves of the agonist (prime mover) has been shown to improve motor performance by enhancing somatosensory input prior to muscle activity. Maioli and colleagues [28] investigated the effects of acupuncture upon upper-limb Motor-Evoked Potentials (MEPs), elicited by transcranial magnetic stimulation of the primary motor cortex in humans. They reported that the simple insertion of the needle is an adequate somatosensory stimulus to induce a significant modulation of MEP amplitude, the sign of which is specific to the investigated muscle and to the point of needle insertion. It has been demonstrated that when applying electroacupuncture or electrical stimulation to induce a motor response, it is vital that parameters creating a motor response that mimics a voluntary muscle contraction are used [81,82]. De Brito, et al. [81] demonstrate that peripheral electroacupuncture or electrical stimulation that is sufficient to induce a motor contraction (30 Hz and 100 Hz) increased corticomotor responsiveness (P = 0.002), whereas stimulation at 10 Hz did not.
It has been shown that the effect of acupuncture on muscle function is brought about through enhancing functional connectivity between the bilateral primary motor cortex, the cerebellum, and the primary somatosensory cortex [83-86]. The motor cortex forms part of the cerebral cortex and is made up of the primary motor cortex, which is responsible for initiating motor movement, and the premotor and supplementary cortex, which are involved with aspects of planning, initiating, and selecting the correct movement.
Assessment of the effect of acupuncture on the motor cortex has primarily involved animal studies or post-stroke patient studies (see Table 1). Functional Magnetic Resonance Imaging (fMRI) and transcranial magnetic stimulation (TMS) have been used to assess the effect of acupuncture on brain activity. While fMRI show real-time hemodynamic changes in brain activity during and following acupuncture, TMS measures changes in the excitability and inhibition of the human motor cortex during and following acupuncture. Several studies [87-91] have shown that there is a significantly greater activation change in the motor-related area, improved blood flow to ischemic areas, and enhanced stroke recovery following true acupuncture compared with sham acupuncture. Yang, et al. [27] used TMS to investigate the neuroplasticity changes in the human motor cortex induced by acupuncture. The acupuncture treatment consisted of 10 acupoints (LI-4, LI-10, LI-11, SJ-5, ST-36, SP-6, GB-34, and the last three Baxie points). They found that acupuncture induced a significant modulation of Motor-Evoked Potential (MEP) amplitudes of the motor pathways that depart from the contralateral primary motor cortex. Changes in MEP amplitude also occur in the ipsilateral primary motor cortex following acupuncture. Sun and colleagues [25] used TMS to explore the effect of ST-36 and sham points on motor cortical excitation and inhibition on 20 healthy volunteers. They found that acupuncture at ST-36 significantly increased motor cortical excitation and reduced motor cortical inhibition with no significant difference from baseline in the sham acupuncture group. Furthermore, deqi sensation was correlated to Motor-Evoked Potential (MEP) amplitude. Like Yang, et al. [27] Sun, et al. [25] also found that when acupuncture was applied to the left-sided ST-36 acupoint, the effects of acupuncture influenced the excitability of the ipsilateral and contralateral hemisphere. In 2019 He, et al. [92] undertook a study to determine whether the changes in the cortical excitability before, during and after acupuncture are time dependent. They used acupoints LI-11 and SJ-5 in the left arm. Like earlier studies [25,27], they found that changes in the MEP amplitudes occurred on both the contralateral and ipsilateral sides. The finding from the TMS studies agrees with fMRI studies that show acupuncture exerts an effect on the supplementary motor area [28,93,94].
Table 1: Characteristics of the studies that assessed the effects of acupuncture on the primary somatosensory and primary motor cortex connectivity. View Table 1
Strength training forms an integral part of an athlete's preparation for competition, return to sport following injury, or the prevention of injuries [95]. Muscles in athletes who partake in regular intensive exercise programs undergo major adaptive changes, which include increased muscle mass, mitochondrial contents, and respiratory capacity of the muscle fibers. The main metabolic adaptation that occurs within the muscles following intense exercise is a slower utilization of muscle glycogen and blood glucose, a greater reliance on fat oxidation, and less lactate production during exercise of a given intensity [96]. Several studies [97-100] have demonstrated that during intensive exercise, athletes who have received acupuncture are able to work harder and longer at a near maximal intensity and will produce a higher level of lactate. Furthermore, our previous research [101] found strong evidence showing that acupuncture is significantly more effective than no treatment or sham acupuncture in enhancing lactate removal following intense exercise thus demonstrating that following acupuncture, athletes will recover much quicker from symptoms of delayed onset muscle soreness. These findings can be explained by the effect of acupuncture on the release of carnitine, glutathione, and creatine into circulation and mitochondrial cells in the muscles. During energy metabolism, carnitine plays a major role in transporting long-chain fatty acids into mitochondrial cells [102]. Like carnitine, creatine helps to energize cells, particularly muscle cells, and helps to build muscle mass. Both creatine and carnitine will help accelerate recovery, improve athletic performance, and increase energy production. Animal and human studies [130-106] have demonstrated a significant increase in carnitine levels following acupuncture compared with control (see Table 2). In 2017 Lin and colleagues [107] investigated the metabolic basis of meridian specificity using proton nuclear magnetic resonance. They used points on the Stomach meridian (ST-2, ST-21, and ST-36) and the Gall Bladder meridian (GB-14, GB-24 and GB-34) and found that EA stimulation produced a significant change in energy metabolism. Stimulation of points along the Stomach meridian produced a marked metabolic increase in glycolysis, while stimulation of points along the Gall Bladder meridian produced a significant change in glutamine and glutamate. Lin, et al. [107] found that there was an increase in lysine and creatine levels following acupuncture. Lysine and methionine are essential amino acids that allow the body to naturally produce carnitine and creatine [108]. Overall, the findings indicated that EA significantly induced a change in energy metabolism with an enhanced aerobic capacity through altered levels of the Krebs cycle intermediates (glucose, lactate, fumarate, citrate, α- KG, and ATP).
Table 2: Characteristics of the studies that assessed the effects of acupuncture on carnitine, creatine, glutathione levels and oxidative stress. View Table 2
A few studies have demonstrated that high-intensity exercise led to an over production of Reactive Oxygen Species (ROS) in muscle tissue, which in excess lead to a decrease in mitochondria respiratory capacity and sarcoplasmic reticulum integrity [109-111]. Oxidative stress is associated with tissue inflammation, fatigue, muscle injury, impaired recovery after high- intensity exercise, and reduced immune function [112-117], all which may impair athlete performance [112]. Mitochondrial cells are particularly vulnerable to oxidative damage during and following intense exercise. With oxidative stress, mitochondria membranes are seriously impaired, and in general, a reduction of mitochondria biogenesis is reported [118]. Investigation to identify micronutrients and natural compounds that can prevent or attenuate exercise- induced oxidative stress and inflammation [119] continues. There is growing evidence that has demonstrated that acupuncture reduces oxidative stress in different conditions [120-125]. Carnitine and glutathione are antioxidants that have a protective role in reducing the overproduction of free Reactive Oxygen Species (ROS) within mitochondrial cells, which in turn reduce damage to muscle cells during intensive exercise. Carnitine preserves the integrity of the mitochondrial membrane by reducing the accumulation of reactive oxygen species, it reduces the production of lactate [126], and allows the mitochondrial cells to utilize energy more efficiently. Shin [105] and Kim, et al. [106] found that when manual acupuncture and electroacupuncture are applied, carnitine levels significantly increased, compared with a sham acupuncture group. In 2007 and 2011, Toda [103,104] demonstrated the effects of EA and MA at ST-36 and ST-41 on carnitine and glutathione levels in the circulation and muscles. Results showed that carnitine levels in muscle tissue following MA were significantly higher than in the EA and control groups, with no significant difference seen between EA and control group. With regards to glutathione levels in muscle tissue, there was no significant difference between EA and MA groups and both groups were significantly higher than the control group [103]. Ma, et al. [99] used a Nuclear Magnetic Resonance- (NMR-) based metabolomics approach to investigate the antifatigue effects of acupuncture on healthy young male athletes who had done exhaustive physical exercises. It was observed that during strenuous exercise there was an increase in reactive oxygen species, enhanced glutathione synthesis along with the production of 2-hydroxybutyrate. 2-Hydroxybutyric acid, also known as alpha-hydroxybutyrate, is a marker that relates to oxidative stress. 2-hydroxybutyrate catabolizes L-threonine or synthesizes glutathione. Ma, et al. [99] found that after the treatment with acupuncture, the levels of 2-hydroxybutyrate, 3-hydroxyisovalerate, lactate, pyruvate, citrate, dimethylglycine, choline, glycine, hippurate, and hypoxanthine were recovered at an accelerated speed in the acupuncture group in comparison with the control group.
Normal levels of Ca2+ are important for vital processes within cells, for example, contraction of muscles, cell proliferation, gene expression, secretion of hormones and neurotransmitters, exocytosis, and chemotaxis. A significant accumulation of Ca2+ in mitochondrial cells can be very toxic leading to mitochondrial destruction, aerobic energy metabolism dysfunction, and inhibition of ATP synthesis [127-131]. In 2005 and 2008 Gao [100,102] and colleagues observed the effects of MA and EA on antioxidant enzyme and Ca2+(-) ATPase activities and Ca2+ content in mitochondria and the sarcoplasmic reticulum of skeletal muscle cells in rats following acute swimming exercise. They found that the Ca2+-ATPase activity value was significantly higher in the EA and MA group compared with the swimming group with the highest value in the MA group. Furthermore, Ca2+ was significantly higher in the EA and swimming groups than in the control and MA groups indicating that MA was more effective at reducing the accumulation of Ca2+ resulting from intense swimming. These findings can be explained by the fact that they found superoxide dismutase, glutathione, and glutathione peroxidase activity to be significantly (P < 0.05 P < 0.01) higher in the MA group than in the other groups.
The purpose of this review was to determine the underlying physiological mechanisms in which acupuncture enhances muscle strength. The overall finding of this review indicates that acupuncture has an acute effect on enhancing muscle strength similar to the PAPE phenomena. Acupuncture has a chronic effect on improving muscle strength by reducing the build-up of oxidants, enhancing glycolysis within mitochondrial cells, and improving connectivity within the central nervous system. Furthermore, MA is more effective than EA at increasing carnitine, creatine, and glutathione levels, reducing the accumulation of Ca2+ and enhancing the respiratory capacity of mitochondrial cells in muscles. ST-36, GB-34, LI-4 and LI-10 (see Figure 2) were found to be the most common points used to enhance the connectivity of the primary somatosensory cortex and the primary motor cortex to induce a strong motor-evoked potential. ST-36 was the primary point used to slow the utilization of glycogen, enhance pyruvate oxidization, and reduce the risk of damage to mitochondrial cells during intense exercise.
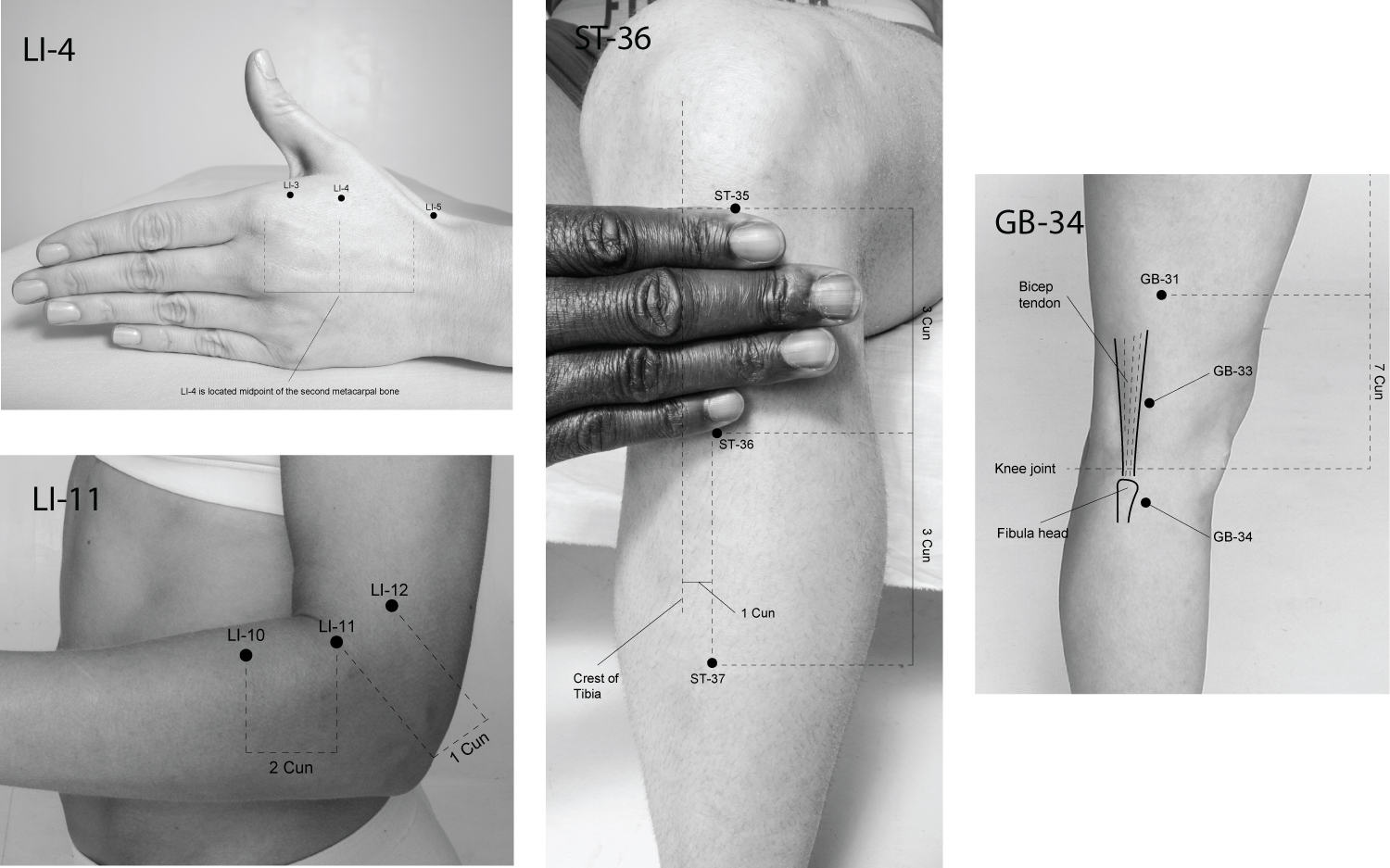 Figure 2: The location of common points to improve muscle strength in humans.
View Figure 2
Figure 2: The location of common points to improve muscle strength in humans.
View Figure 2
Acupuncture enhances muscle strength in several ways, which include improving the efficiency in which mitochondrial cells function, reducing inflammation, and releasing antioxidants thereby reducing the build-up of reactive oxygen species and damage to cells. Furthermore, acupuncture produces a strong motor-evoked potential. Most studies in this review were undertaken on post-stroke patients, therefore further studies with a large sample size are required on healthy athletes to help determine the underlying physiological effect of acupuncture on improving muscle strength in sports.
The limitations were that studies that were not in English were not included in this review and the methodological quality of each of the RCTs and systematic reviews was not assessed due to the nature of this narrative review.
The author declares that they have no conflict of interest.