Extracellular vesicles (EV) are small membrane bound vesicles which are capable of delivering biomolecules to neighbouring recipient cells or more distant cells, where they induce various signalling cascades and subsequently alter their metabolism. These EVs may include apoptotic bodies, microvesicles, and exosomes, which have emerged as important mediators of cell to cell communication in normal physiology and pathological status. EVs have been widely documented in vascular development, growth, and maturation and their potential therapeutic application in angiogenesis related diseases is drawing an increasing engrossment. The effect of endurance exercise on circulating vesicle dynamics and their impact on surrounding endothelial cells is of much interest now days. The impact of persistence workout on circulating vesicle dynamics and their effect on surrounding endothelial cells may be of considerable significance. Different workout intensity interferes with the time path and appearance of platelet microvescicles (PMV) and endothelial-derived microvesicles (EMV) inside the blood circulation via hemodynamic and altered biochemically-associated mechanisms, and that microvesicles formed in the course of workout which might stimulate endothelial angiogenesis in vitro. It is to be observed that whether or not low, mild or excessive intensity of endurance exercise are able to generate equal results on microvesicle formation. Furthermore our fundamental concern is to perceive the upgradation of sports activities on overall performance by means of enhanced microvesicle formation.
A-V: Arteriovenous; ATM: Ataxia Telangiectasia Mutated; BA: Brachial Artery; CAD: Coronary Artery Disease; CCl2: Chemokine c-c motif ligand 2; CON: Resting Control Trial; BF: Blood Flow; CRP: C-Reactive Protein; Dll4= Delta-like 4; ECM: Extracellular Matrix; eNOS: Endothelial Nitric Oxide Synthase; EDTA = Ethylenediaminetetraacetic Acid; EDV: End Diastolic Volume; EMV: Endothelial Microvesicle; ERK1/2: Extracellular signal-related kinase 1 and 2; ETS1: Avian erythroblastosis virus E26 homolog-1; ESCRT: Endosomal Sorting Complexes Required for Transport; ESV: End Systolic Volume; exMVs: Circulating microvesicles during exercise; FA: Common Femoral Artery; FBS: Foetal Bovine Serum; FSC: Forward Scatter; Hb: Haemoglobin; Hct: Haematocrit; HI: Heavy Intensity Trial; HR: Heart Rate; HUVEC: Human Umbilical Vein Endothelial Cell; IL: Interleukin; IL-3R: Interleukin-3 receptor; MAP: Mean arterial pressure; MI: Moderate intensity trial; MMPs: Matrix metalloproteinases; MTT: Thiazol blue tetrazolium bromide; MV: Microvesicle; NO: Nitric Oxide; NotchR: Notch Receptor; PMV: Platelet Microvesicle; PPO: Peak Power Output; PPP: Platelet-Poor Plasma; PS: Phosphatidylserine; Q̇: Cardiac Output; Rac1: Ras-related C3 botulinum toxin substrate 1; ROS: Reactive Oxygen Species; rMVs: Circulating microvesicles at rest; rpm: Revolutions Per Minute; SD: Standard Deviation; EM: Standard error of the Mean; SR: Shear Rate; SSC: Side Scatter; SV: Stroke Volume; TF: Tissue Factor; TIMPS: Tissue Inhibitor Of Metalloproteinases; TNF-α: Tumour Necrosis Factor alpha; uPA: Urokinase Plasminogen Activator; uPAR: Urokinase Plasminogen Activator; VEGF: Vascular Endothelial Growth Factor; V̇CO 2 : Carbondioxide Production; V̇O 2 : Oxygen Uptake; V̇O 2max : Maximal Oxygen Uptake; VT 1 : Ventilator Threshold 1
Extracellular vesicles (EVs) are spherical vesicles surrounded by membrane bound structures produced in an evolutionarily conserved way in cells ranging from prokaryotic organisms to better eukaryotic organisms as well as in plant cells. The significance of potency of the EVs lies in their capacity to transfer information to other cells thereby stimulating recipient cell function. EV-mediated signals can be conveyed through different biomolecules including proteins, lipids, nucleic acids and sugars. The packaged information provides both protection and the delivery of multiple messenger molecules even to sites remote to their vesicular origin [1]. In the late 1960s, the first study described the mechanism of release of EVs from either platelets or chondrocytes. Later studies revealed that both normal and neoplastic mammalian cells were capable of secreting vesicles containing 5’-nucleotidase enzymatic activity that ranged in size from 40 to 1000 nm and originated directly from the plasma membrane and in the year 1981 researchers coined the vesicles as ‘exosome’ [2]. Some studies have analysed the transferrin receptor (TfR) was recycled from the plasma membrane of maturing reticulocytes by selective endocytosis through internalization into a so-called endosome (currently known as multivesicular bodies, MVBs) and then subsequently secreted from the cell [3,4]. The first study showed that EVs contain functional microRNAs (miRNA) capable of regulating target cell gene expression and this fact generated special interest among the researchers [5]. Since then several works have followed up to discover the role of EVs as a medium for intercellular communication and the interest in the field increased exponentially [6,7].
It has been attempted to assess the relationship between the benefits of exercise on health and the change in extracellular vesicle formation kinetics. Physical exercise introduces systemic physiological challenges capable of acutely disrupting cell homeostasis. Cells secrete extracellular vesicles under normal circumstances as well as in response to diverse stimuli for the purpose of cell communication and tissue homeostasis. Here we are doing this project to explore that physical activity is associated with the release of nano-sized EVs into circulation and different properties of microvesicles expressing after an incremental exercise protocol. Certain biochemical parameters changes during exercise such as blood pressure, stroke volume, cardiac output, hormonal level, cytokine profile resulting in interweaving extracellular vesicles function.
Most of the early examinations regarding underlying mechanisms of generation of extracellular vesicle were based on Platelet microvesicles (PMV), nonetheless, recent works have focused on the agonists responsible for the formation of Endothelial microvesicles (EMV) [7]. An exhaustive and comprehensive characterization of EV content, leading to the creation of specialized databases [8,9] such as EVpedia that records the bioactive molecules and markers observed within these vesicles. Vesicle content specifically reflects the vesicle’s localization, cellular origin, and mechanism of secretion [10]. Depending upon such data, microvesicles have been classified into three broad categories: Those are exosomes, microvesicles and apoptotic bodies. A set of proteins is commonly found in EVs, which are associated with vesicle trafficking and biogenesis, such as tetraspanins (CD81, CD9, and CD63) [11] specific stress proteins (HSP90 [heat shock proteins]), members of the Endosomal Sorting Complexes required for Transport complexes (Tsg101, Alix), proteins involved in membrane fusion (Rabs, ARF6), and signalling proteins and several others [12]. More significantly, EVs also carry membrane receptors on their surface that bears characteristic of the cells from which they have been generated. In addition to their surface molecules, EVs has found to carry important soluble mediators, such as cytokines, growth factors, and transcription factors [13]. The lipids that are generally enriched in EVs are sphingomyelin, cholesterol, phosphatidylserine, ceramide, and glycosphingolipids, which confer the raft domains. Phosphatidylserine represents one of the characteristic EV markers in the membrane that is expressed on the outer surface. Interestingly, an absence of phosphatidylserine has been recently identified in microvesicles [14]. Besides having proteins and lipids, EVs can also incorporate genetic material, such as small and long, coding and noncoding RNA (mRNA, miRNA, and lncRNA), and other cytosolic components and molecules [5,15]. Some studies have also reported the presence of genomic and mitochondrial DNA in the hollow pocket of EVs [16].
The lipid-bilayer EVs help to encase their genetic cargo and protect it from enzymatic digestion [17]. EVs may, therefore, serve as nucleic acid delivery vehicles, representing a new mechanism of genetic exchange between various cells [18]. For example, several studies have showed that enclosed mRNA can be exchanged between cells and can induce the reprogramming of hematopoietic progenitors and ECs. EVs can directly shuttle RNAs and transcription factors, which are capable of inducing phenotypic changes in the target cells [18]. Thus, the EV-mediated transfer of genetic material may directly modulate the phenotype and behaviour of the recipient cells.
Size varies from 30 to 120nm, cup shaped particle. Originates mainly by exocytosis from Microvesiculated Body following underlying Pathways: ESCRT-dependent, Tetraspanin-dependent, Ceramide-dependent. Marked with specific proteins like: CD81, CD63, Alix, Tsg101 [19]. General composition includes protein, lipid, coding RNA, noncoding RNA, DNA [1].
Size of microvesicles varies from 100 to 1000 nm, and these are particle formed by budding from plasma membrane following various pathways. Presence of protein markers can be observed e.g. Selectins, Integrins, CD40. Microvesicles are mainly composed of protein, lipid, cell organelles, coding RNA, noncoding RNA, DNA [1].
Size varies from 800 to 5000 nm, heterogeneous in nature. Originated by budding from the plasma membrane due to apoptosis-related pathway. Presence of specific protein markers like Caspase 3, Histones. It is generally composed of Cell organelle, proteins, nuclear fractions, coding RNA, DNA [20].
An intact membrane of a platelet presents asymmetric phospholipid distribution with negatively charged varieties, such as phosphoserine (PS), located on the inner leaflet and neutral phospholipids on the outer membrane leaflet. For maintaining proper cellular function and regulating vascular haemostasis, this irregular membrane distribution is required and can be altered when necessary. The abundance of PS on the cytosolic leaflet of the cell membrane is regulated by the amino phospholipid translocases, flippase and floppase. Flippase are ATP-dependent proteins that transport PS to the inner membrane, whereas the floppase can disturb membrane asymmetry by outward translocation of this membrane lining phospholipid [21]. The intra-cellular pathways that eventually result in vesicle formation are actually depending on the stimulatory agonist and cell type (platelet or endothelial). The activation of calcium-dependent proteins was initially suggested as a central mechanism in extracellular vesicle formation, as observed, an increase in PS translocation, and MV release from platelets when their cytoplasmic Ca 2+ concentrations were increased through agonist stimulation [22].
High shear stress, IL-6, thrombin, noradrenalin act as the positive effectors where as low shear stress, IL-1α, TNF α act as negative effectors.
Endocytosis is a complex process that includes several molecular pathways involved in the cellular uptake of EVs. The different endocytic pathways comprises of clathrin-mediated endocytosis, caveolin-dependent endocytosis, macropinocytosis and phagocytosis. The process of clathrin-mediated endocytosis involves cellular internalization of molecules through the assembly of clathrin-coated vesicles [23]. These vesicles distort the membrane followed by triggering an inward budding of the plasma membrane followed by fusion with the endosome [24]. Whereas caveolin-dependent endocytosis is a clathrin-independent endocytic process involving plasma membrane invaginations called caveolae, which are ultimately internalized by the cell [25]. Macropinocytosis leads to the formation of membrane ruffles that pinch off to the extracellular space, which in turn is surrounded by an area of extracellular fluid subsequently internalized by the target cell [26,27]. Phagocytosis is the process by which the cell uses the plasma membrane to engulf large particles, thereby creating a membrane-bound vesicle called a phagosome [28-30]. All of these endocytic pathways have been shown to play role in the cellular uptake of EVs [23,31,32].
Subsequently, upon its binding, EVs may trigger intracellular signalling pathways through a simple interaction with the surface receptors or ligands of target cells or by the undergoing internalization. Studies showed that direct fusion of EVs with the plasma membrane may be limited to acidic pH (~5) [33]. The key impact of the microenvironment’s pH suggests that the differences in the electrostatic charges between EVs and the plasma membrane of the cells should be considered in relation to the physiological roles of EVs [34]. The steady-state level of EVs in circulation reflects a balance between the EV generation and their clearance from circulation is most likely due to retention and uptake in target organs [35].
Many researchers have tried to culture inner vascular cells, which were initially supposed to be none other than fibroblasts by some scientists in the 1960’s. It was represented as a polygonal cell, expressing antigens of the ABO blood system. Today it is known that endothelial cells originate from haemangioblast cells and form a single layer of specialised epithelial cells that cover the innermost lining of vessels, acting as an active semi-permeable barrier between the blood and the surrounding tissues [1].
Researchers have found that microvesicle may suppress angiogenesis by down regulating the endothelial expression of vascular endothelial growth factor (VEGF) receptors coupled with increased superoxide anion production, and pathological levels of EMVs which may exhibit paracrine effects resulting in decreased nitrous oxide (NO) bioavailability through the production of reactive oxygen species by target endothelial cells.
Several studies have demonstrated that circulating MVs obtained from patients with a previous myocardial infarction reduced acetylcholine-induced endothelial mediated dilation in situ . Circulating vesicles may have some detrimental effects on vascular endothelial cells but has proven to be effective in inducing some positive responses. Experimentally-induced limb ischemia increases the concentration of MVs thereby indicating the existence by expressing skeletal muscle and endothelial antigens within local muscle homogenates and these MVs potentiate progenitor cell differentiation into an endothelial phenotype and subsequent angiogenesis within limbs. There are possible discrepancies between the roles of MVs which may be attributed to the events leading to their formation under pathological and non-pathological conditions [6].
Therefore, the purpose of the study is to characterize the impact of modulating exercise intensity on the time course of PMV and EMV concentrations during exercise and recovery and investigate the potential effects of circulating microvesicles produced during exercise on angiogenesis, proliferation, and migration of human endothelial cells.
The purpose of this systematic review was to explore the exercise intensity induced changes in the appearance of circulating microvesicles and collect some scientific evidences. The literature search held through electronic databases and articles in scientific journals such as Google Scholar, SCI-HUB, Sports Medicine and Journal of Medicine and Science in Sports, etc. The main databases used were: PubMed, Research Gate, World Health Organization (WHO), and National Center for Biotechnology Information (NCBI). The following keywords uses for our literature review search: platelet microvesicles; Microparticles; exosomes; shear stress; endothelial cell; angiogenesis; exercise intensity; microvesicle-endothelial cells interactions; formation and uptake; proangiogenic cells; tubular formation; haemodynamic. In addition, references of retrieved studies were manually screened to obtain relevant studies.
The articles retrieved were based on the following inclusion criteria:
• Studies that were conducted to determine the formation, shedding, uptake mechanism of microvesicles published in the English language;
• Studies in which microvesicle formation are used in combination with exercise intensities;
• Studies in which angiogenic properties of microvesicles, other functions as well; and
• Experimental and clinical studies with full texts
I excluded studies based on the following criteria:
• Studies in which microvesicles were not used;
• Studies in which micropraticles were used in combination with other agents;
• Studies that made use of other forms of vesicles from other origin such as uterus, neurons etc.; and
• Conference abstract, simulation studies, case reports, letters, editorials, unpublished data, articles without full texts, and non-English articles.
Data from each eligible study were extracted by me. The following information was obtained: Author name, year of publication, subject, organ (or tissue) of interest. Furthermore, the main outcomes were summarized and included.
All retrieved articles from electronic as well as manual searches were recorded. Thereafter, duplicates were removed. Afterwards, I independently reviewed the titles and abstracts of the retrieved studies for eligibility. Studies were then selected based on the predetermined inclusion and exclusion criteria.
The PRISMA flow diagram showing my search results is presented in Figure 1. My initial search gave a total of 308 records, with the breakdown as follows: 305 records from electronic databases and 3 records obtained through a manual search. From these figures, 220 records were retained after removing duplicates. Following careful examination and 8 screening of their titles and abstracts as well as the application of the inclusion and exclusion criteria, a further 108 records were excluded. Based on screening, 83 records without full text from 112 records were excluded. The full texts of the remaining 29 records were assessed. A total of 10 of these full text articles were excluded for non-English language, not having full texts, not controlled study, or not being a research article. Thus a total of 19 studies were included in this systematic review.
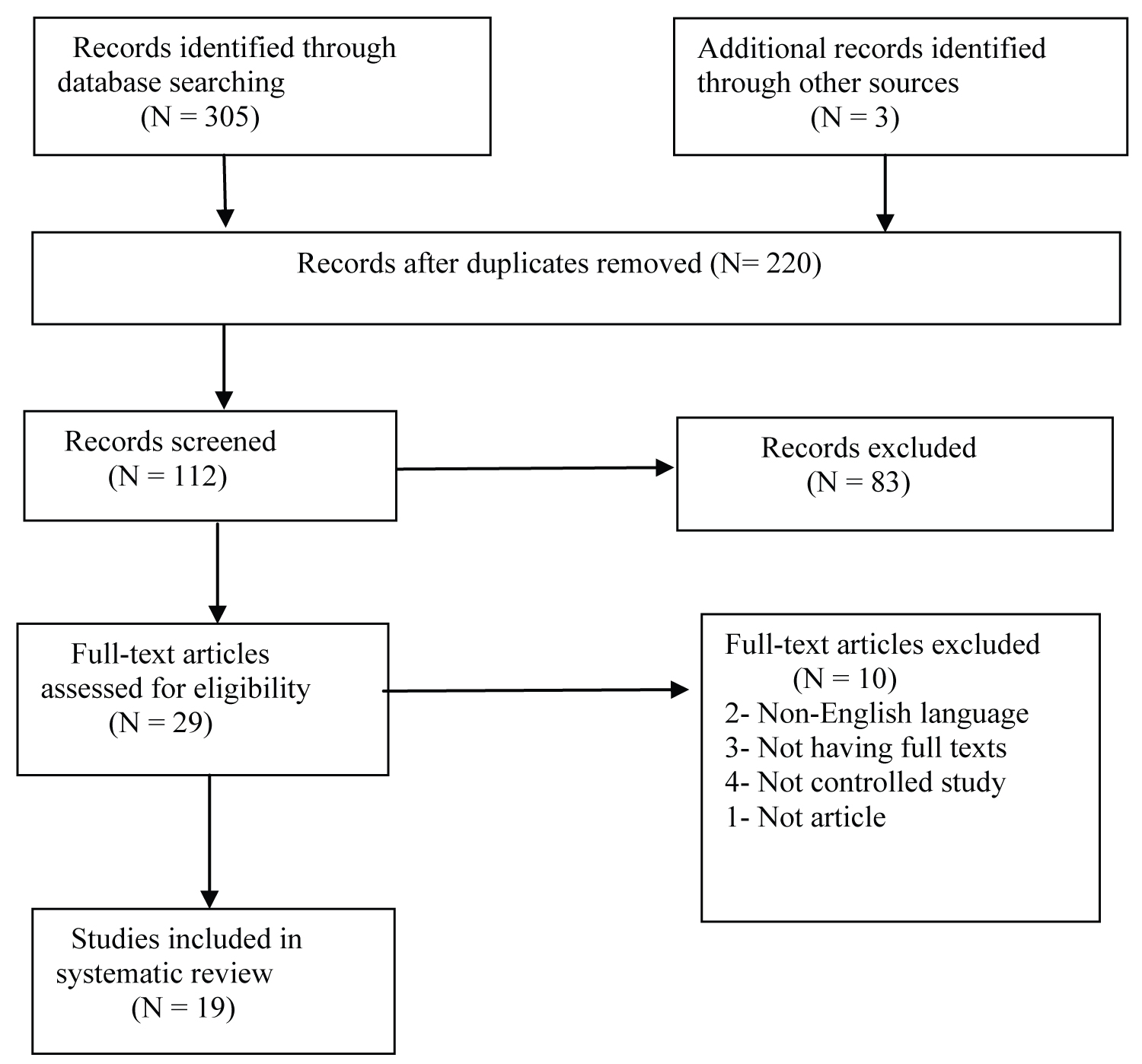 Figure 1: PRISMA flow diagram for the selection of included article.
View Figure 1
Figure 1: PRISMA flow diagram for the selection of included article.
View Figure 1
The experiment was designed to detect the appearance of microvesicle in the circulation during different grades of exercise (moderate and heavy) and at recovery-
• To clarify the dynamics of the appearance of microvesicles in the blood stream in response to dynamic exercises of two intensities.
• To study the effects of circulating microvesicles, induced during exercise on endothelial proliferation, chemotaxis and angiogenesis in vitro [36].
The experiment was strictly conducted in a thermally neutral environment for the accurate results. At the beginning participants started cycling at 80 rpm with three progressive repetition (5 min of easy paddling proceeding with 5 mn of rest). First repetition started with 3 min of warm up at 60W that was increased by 30 W/min until maximal oxygen consumption was reached. The following repetition directly started with 1 step at 60W with incremental load advancement.
It will be considered as attainment of maximal oxygen consumption will if four criteria have matched:
• A respiratory exchange ratio greater than 1.10
• Attainment of age-predicted maximal heart rate (HRmax) [210 – (0.65 × age) ± 10%],
• Increase in oxygen uptake (VO 2 ) lower than 100 ml with the last increase in work rate, and
• Inability to maintain the required pedaling frequency (70 rpm) despite maximum effort and verbal encouragement [37].
Data that has been retrieved, reveals that the estimated lower limb blood flow (BF) increased during cycling as a function of exercise intensity, and remained elevated at 5 min post-exercise with more pronounced values in heavy intensity trial (HI) reflecting the augmented metabolic demand of the lower limbs. Correspondingly, early post-exercise recovery common femoral artery (FA) and mean shear rate (SR) was higher than baseline, with substantially greater values post heavy cycling compared to moderate intensity. This BF and SR response occurred mostly through a rise in blood velocity to the lower limbs, with arterial vasodilation playing a smaller role [37]. During exercise, brachial artery (BA) vasodilation occurred and the mean blood velocity was increased, with a resultant rise in BA BF during exercise and into early recovery. The higher BF to upper limbs during heavy exercise, in comparison to moderate exercise, was mostly driven by changes in blood velocity, as no differences in BA diameter were observed between exercise intensities. Similar to velocity changes, mean BA SR was augmented during cycling as a function of exercise intensity, and was still elevated above baseline by 5 min of post heavy exercise recovery, returning to resting values by 20 min.
At the start of exercise, HR readily increased in relation to exercise intensity and remained elevated throughout the exercise bouts, returning to baseline by the 20 th min of recovery after both exercise protocols, whereas SV increased during cycling independent of exercise intensity and returned to resting values within the first 5 min of recovery. This resulted in an elevated cardiac output (Q̇) throughout cycling in accordance with exercise intensity. In the first 5 min of recovery after heavy exercise, Q̇ remained greater than baseline and in comparison to resting control trial (CON), whereas it returned to values similar to rest following the moderate intensity trial (MI) protocol. Similarly, mean arterial pressure (MAP) increased during exercise reaching higher values during the HI compared to the MI at 40 min but quickly returned to baseline at the end of exercise. The MAP at 5 min of recovery, however, was lower during the HI compared to MI reflecting a tendency for post-exercise hypotension after prolonged heavy exercise [38].
After analysis of the experimental data, it was evident that [EMV] was stable throughout the 4 h of CON and did not change in MI and HI trials. However, a significant Time x Condition interaction was observed for [PMV], which increased with heavy exercise. A biphasic PMV response was apparent during HI trial; increasing by ~2 fold during 30 and 60 min of heavy exercise subsequent to which, a declination occurred, however a second rise above baseline was evident at 60 min post heavy exercise [39]. Plasma volume corrections did not eliminate any changes in PMVs during and after heavy exercise [37].
Haematocrit (Hct) increased throughout the heavy intensity bout, with increases during MI were evident at the end of moderate exercise and 20 min of recovery [40,41]. Concomitant changes in blood haemoglobin (Hb) concentration were observed at all exercise time points during both cycling intensities and returned to baseline during recovery [42]. Plasma volume was reduced during both moderate and heavy cycling, and returned to baseline between 20 and 40 min after exercise [43].
As expected, heavy intensity exercise (performed above the ventilator threshold [VT1]) elicited higher metabolic demands amongst the active muscles as evidenced by 5-6 fold increases in blood lactate concentrations throughout the exercise session. This response was proportional to exercise intensity, with moderate exercise inducing a small changes in blood lactate, although this was significant at the 30 min exercise time point. After exercise, lactate was similar to baseline, but its concentration was lower throughout the first 1.5 h of recovery from moderate exercise compared to heavy [44].
Due to high shear stress platelet derived microvesicle may show procoagulent activity [45]. Exposure of the negative charge derived from membrane phoshatidylserine phospholipid [46]. This leads to binding of several activated coagulation factors to the membrane enabling the formation of the prothrombinase complex followed by thrombin formation [47]. The procagulant activity of microvesciles contributes to the activation of coagulation occurs due to exercise [48].
Plasma noradrenaline concentration rose from baseline levels during heavy cycling, (Time x Condition interaction), with greater values being observed throughout the heavy cycling period compared to control, and at 30 min of exercise compared to moderate cycling. Noradrenaline concentration returned to baseline within the first hour of recovery after heavy exercise and remained similar to baseline until the end of the recovery period. No changes from baseline were observed during CON and MI. By the end of 1h of heavy cycling IL-6 concentration increased compared to moderate intensity exercise and resting control and values remained elevated above baseline throughout the recovery period [49]. Interestingly IL-6 concentrations also increased from baseline in the CON and MI during the 3 rd and 4 th h of the protocol, so that at the end of the recovery period no differences were observed between trials [50]. It is experimentally proven that contracting skeletal muscle was one of the main sources for IL-6 in the circulation in response to exercise [51,52]. Different mechanisms during intensified exercise such as muscle damage [53], extensive muscle contraction may lead to formation of reactive oxygen species (ROS) [54] and include glucose unavailability [55]. Effects of IL-6 as an anti-inflammatory have been shown to affect liver and different population of leukocytes [56].
Since shear stress, noradrenaline, and IL-6 have been shown to act as agonists for PMV release in vitro , within-subject regressions were performed to identify whether haemodynamic and biochemical variables could explain the changes observed in PMVs with exercise. A modest yet significant relationship was found between PMVs, BA SR and noradrenaline concentrations when all time-points were investigated. Further analysis revealed that [PMV] moderately correlated with BA SR and, to a lesser degree, noradrenaline levels prior and during exercise. Furthermore, [PMV] were also related to Q̇, and to estimated leg BF during exercise. PMVs displayed no significant correlation with IL-6 under these conditions [45].
EVs are produced by different cell types, and they can regulate angiogenesis by stimulating or inhibiting it. These effects are highly dependent on the EV content and surface molecule expression, which can be strongly regulated by the stimulus used to induce EV production. Angiogenesis is a dynamic and strictly regulated processes in which EC continuously interact with their environment through various paracrine factors and cell-to-cell and cell-to matrix interactions. The angiogenic potential of the vesicles shed by EC was first documented in 2002 [57]. The EVs released by EC contain β1 integrin and express the enzymatic activity matrix metalloproteinase matrix metalloproteinase- 2 and matrix metalloproteinase-9. The EVs were capable of promoting EC invasion and capillary-like structure formation in vitro [57]. In addition, the stimulation of VEGF (vascular endothelial growth factor) and FGF-2 (fibroblast growth factor-2) led to an increase in the amounts of both the active metalloproteinase and a number of proenzyme forms in vesicle-associated compartments. Recently, it was shown that the EVs produced in response to interleukin-3 stimulation promoted angiogenesis by transferring of miR-126-3p and pSTAT5 into the recipient EC resulting to a lower Spred-1 level and increased ERK1/2 activation and cyclin D1 transcription [58]. Other micro-RNAs in the EC-derived exosomes, such as miR-214, had been shown to promote angiogenesis both in vitro and in vivo by repressing ataxia telangiectasia mutated (ATM) expression and preventing senescence [59].
Interestingly, it was demonstrated that signalling does not need to be in direct cell contact, as δ-like 4 can be integrated into endothelial exosomes and transferred to the target EC. These δ-like 4 containing exosomes stimulate the formation of capillary-like structures in vitro and in vivo through the internalization of the Notch receptor and their subsequent degradation [60]. The proangiogenic effect of the microparticles were mediated by the interaction of β1 integrin with the target EC, leading to Rac1-ERK1/2- ETS (Ras-related C3 botulinum toxin substrate 1-extracellular signal-related kinase 1 and 2-avian erythroblastosis virus E26 homolog-1) signalling and chemokine c-c motif ligand 2 (CCL2) production [61]. Higher concentration of EC-derived microparticles inhibited angiogenesis in the cultured sections of hearts, resulting eNOS (endothelial nitric oxide synthase) dysfunction [62]. Recently, studies have shown that EVs derived from ECs inhibit microvascular ECs from tubular structure formation in vitro and in vivo . This anti-angiogenic effect of EVs was caused by reactive oxygen species in the NADPH (nicotinamide adenine dinucleotide phosphate) oxidase and Src kinase-dependent family and requires the expression of CD36 in the target EC [63]. The involvement of oxidative stress in the anti-angiogenic effect of EV has also been confirmed by other studies, demonstrating that endothelial microparticles change the acetylcholine-induced endothelial vasodilation and production of nitric oxide (NO) in rat aortic rings and inhibition of angiogenesis by increasing the oxidative stress [64,65].
EC release micro-RNA rich EVs, such as miR-214 and miR-126, which are delivered to the recipient EC and induce pro-angiogenic signalling. EV contains functional matrix metalloproteinases that promote angiogenesis by decomposing the extracellular matrix. Delta like 4 (Dll4) is passed from the EVs to EC and induce the internalization of Notch receptor and formation of the apical membrane. EV carries a tissue factor on its surface, which interacts with β1 integrin and induces Rac1-ERK1/2-ETS1 signalling, leading to the increased secretion of CCL2. EV transports urokinase plasminogen activator (uPA/uPAR) complex, which stimulates angiogenesis by forming plasmin. The phosphatidylserine present on the surface of the EVs interacts with CD36 and induces Fyn kinase signalling, leading to increased oxidative stress and the inhibition of angiogenesis.
Although the present findings explored a number of interactions between exercise, intravascular MVs, and the vascular endothelium etc., they certainly do not cover all aspects of this relatively new research field. Results from these reviews demonstrate the importance of exercise intensity in PMV formation. However, the precise intensity at which significant elevations in MV appear was not determined. Since circulating MVs formed during exercise were initially seen as novel postulants of positive endothelial responses in these studies, the identification of a minimal work rate necessary to bring about increases in blood MVs could be of practical significance for future training related research in the applied ambience. This could be accomplished using maximal incremental test protocols with blood sampling performed at each exercise step. Although, certain restrictions limited the number of blood samples available for MV quantification, and this topic will have to be addressed in future experiments.
The acute influence of circulating MVs formed during exercise on the proliferation and morphogenesis of cultured endothelial cells were also explored through different trial and error, but their importance in vivo and relevance to long term vascular adaptations with exercise remain to be determined in experiments where exercise-derived MVs have to be applied in vivo models, and in which, blockage of the increase in circulating MVs during exercise is to be performed. Reducing the release of PMVs during acute exercise might be possible during testing, using high doses of platelet antagonists, such as acetylsalicylic acid, and could provide further mechanistic insights about PMV formation during exercise and their subsequent effects on endothelial cells. Experiments with chronic inhibition of MV formation might be difficult to perform in humans, but the investigation of long term vascular responses to exercise amongst Scott syndrome patients (a rare congenital bleeding disorder due to defects in platelet plasma membrane remodelling and MV release) may be able to provide the first insight into the importance of acute changes in MVs on chronic endothelial adaptations. This would have clinical relevance amongst Scott syndrome and other patient populations and may ultimately lead to the development of training strategies to optimise the prevention of vascular dysfunction.
There are some prominent instances of using EVs for clinical application. Exploration of other corners about microvesicles is also included in it. EVs of defined cell types may serve as novel tools for various therapeutic approaches:
Pathogens, like helminths (flat- and round-worms), fungi, bacteria as well as parasitic protozoa, including species of Plasmodium, Trypanosoma, Leishmania and Trichomonas , also secrete EVs. Both gram-positive and gram-negative bacteria can release EVs; that are commonly called outer membrane vesicles (OMVs) [66]. Furthermore, pathogen-infected cells can release EVs containing pathogen-specific antigens, as well as from tumour cells, the effects of which have been studied as vaccines in numerous preclinical mouse models. The pros of using EVs rather than whole cells as carriers of MHC-peptides complexes for vaccination (both for immunotherapy and infectious diseases), is that, EVs are more stable upon freezing and thawing than cells, which always undergo a degree of mortality.
EVs are extensively in demand as systems for therapeutic delivery of different drug types. Recent studies highlight the most relevant features of using EVs in targeted drug delivery such as their circulation time, bio-distribution, cellular interactions and the different methods for therapeutic cargo loading and administration [67]. Potential advantages of EV-based drug delivery over the existing synthetic delivery systems (such as liposomes) include decreased immunogenicity and toxicity, increased stability in circulation and tissue, and intrinsic homing abilities. Drugs that could particularly benefit from delivery by EVs are small RNA therapeutics, including miRNA and siRNAs, and anti-inflammatory agents as well as anti-cancer drugs. However, they are incapable of carrying such large, hydrophilic and charged molecules, restricted by the plasma membrane. The observation that EVs released by tumour cells, can transport cytotoxic drugs, such as cisplatin in its native state, from one cellular compartment to another [68]. Accordingly, EVs are considered promising anti-tumour drug delivery vehicles, which help to find a way around the mechanisms mediating chemo-resistance following conventional drug application. For instance, tumour acidity represents a very efficient, though non-specific cause of chemo-resistance, inducing the protonation of the drug and consequent neutralization in the extracellular environment [69]. Drugs transported via EVs may be protected within such acidified microenvironments and, thus, might facilitate the efficient delivery of active drugs into tumour cells in an acidic microenvironment. Micro-environmental acidity has been shown to increase both EV-targeting to the tumour sites and EV-uptake by tumour cells.
Anti-inflammatory drugs such as curcumin or chemotherapeutic agent has made their way through EV mediated transportation.
EVs seemed to play an important role in mediating the therapeutic effects of cells being used as therapeutics, such as mesenchymal stem/stromal cells (MSCs) or endothelial cells. MSCs can be isolated from different tissues, including bone marrow, adipose tissue and umbilical cord blood [70], considered to exhibit pluripotent developmental capabilities (oestrogenic potential). MSCs exert strong immuno-modulating activities such as, suppression the proliferation of mitogen-stimulated T cells, inhibition DC maturation and activation, modulate B cell and NK cell functions, and promote regulatory T cell formation, regulation of pro-inflammatory as well as anti- inflammatory macrophages. Subsequent works have confirmed the protective properties of MSC-derived EVs include kidney regeneration in severe combined immunodeficiency (SCID), treatment of immunological disorders, e.g. graft - versus - host disease (GvHD), Crohn's disease and rheumatoid arthritis [71,72].
Heavy exercise increases the concentration of circulating PMVs during and at 1h post exercise, with no impact on EMVs. This phenomenon depends on exercise intensity, since moderate cycling did not bring about this physiological response. The rise in PMVs during heavy exercise was accompanied by changes in vascular shear stress, and plasma noradrenaline concentration, and both variables appear to explain part of the MV dynamics during exercise. Therefore, this review demonstrates that exercise bouts performed in the heavy exercise domain increase the appearance of circulating PMVs in young healthy men, potentially through the interplay of factors such as elevations in vascular shear stress and circulating catecholamines.