Recently, rare diseases have received worldwide attention. The developing countries have fallen seriously behind in regards to awareness, drug development, diagnosis, and social services. India, which has one-third of the world's rare disease population, has neither accepted definition for RD nor an accurate assessment of the problem. Due to the exorbitant cost of orphan drugs, difficulties in diagnosis and treatments, the Indian government is often in a dilemma as to how to effectively, and efficiently formulate a National Health Policy with a 1.45% GDP healthcare budget for 1.3 billion people.
Indian policymakers want to find out the number of RD and the extent of the population suffering from them. It is not possible to give an adequate answer without understanding the scope to which RD occurs within the general population. A survey was conducted to know the basic knowledge of RD in the general population. The sample survey was obtained from a subset of the population with the criteria arbitrarily set as people with above-average literacy who could be easily reached and expected to understand the proposed study.
A questionnaire was designed to collect information specific to awareness of RDs and ODs. The 6005 people were contacted via emails; 599 responses were received. The results show that the health care professionals appear to have some awareness as compared with non-healthcare professionals, but even among health care professionals, only one third had a rudimentary understanding of RD and OD, whereas three-fourths have virtually no knowledge of RD.
Forty-three percent of health professionals had not seen rare disease patients, and a large percent of practicing physicians had not seen even one rare disease patient in their entire professional practice. It becomes clear from this survey that the most important issues are awareness and diagnosis. This survey is the first of this kind ever conducted and is a tentative systematic first step towards understanding the basic knowledge of rare diseases in India. The results serve as a preamble to complete understanding. Much more must be done to gauge the magnitude of the problems to draft the targeted and effective national health care policy for rare diseases.
India, Health policy, Rare diseases, Orphan drugs, Survey, Awareness
RD: Rare Disease; OD: Orphan Drugs; RDI: Rare Diseases International; IRDIRC: International Rare Diseases Research Consortium; NRI: Non-resident Indian
Rare diseases - diseases that affect a small number of people compared to general populations. Very recently, the phrase "rare diseases" got the attention of the public and scientific community in India [1] (Figure 1). Quite often, RDs are mistaken for neglected or tropical diseases such as TB and Malaria. Many RDs are genetic, and in some cases, the cause is unknown. Currently, there are more than 7,000 known rare diseases [2], which affect an estimated 8-10% of the world population. Unlike neglected diseases, which are localized in certain countries, the RDs have no geographical boundaries.
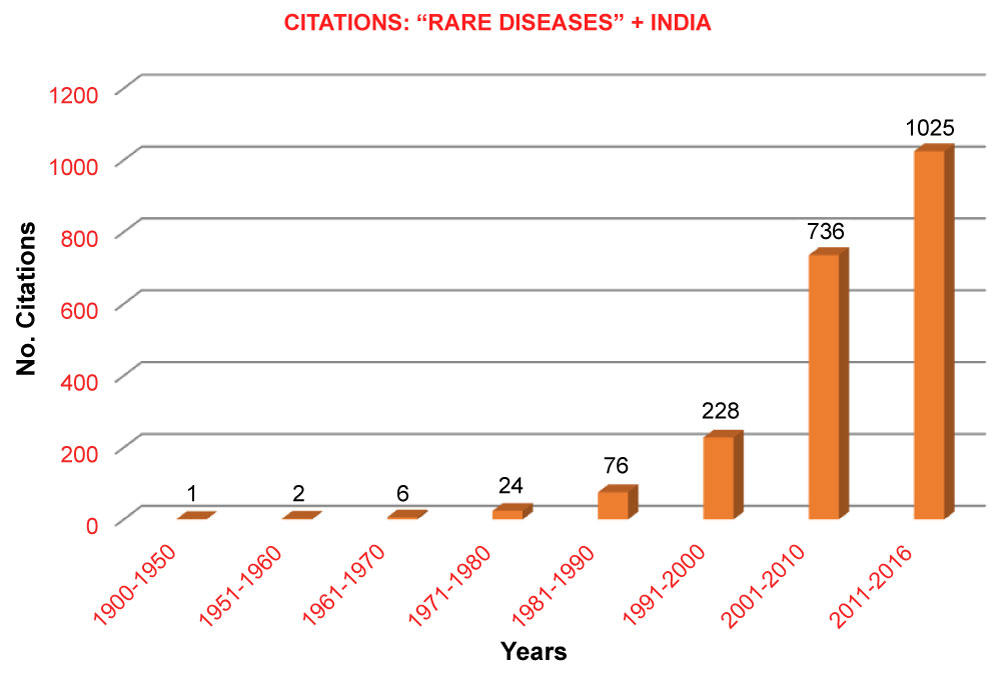 Figure 1: Citations of [Rare Diseases + India] from the PubMed.
View Figure 1
Figure 1: Citations of [Rare Diseases + India] from the PubMed.
View Figure 1
In the early 80s, the rare disease is arbitrarily defined in the USA as a condition, which affects less than 200,000 of the population [3] while, other countries, for example, Europe defined 1 in 2000, Japan defined 1 in 2,500 - a population-based definition. The rarity heightens the isolation and offers limited treatment options. The total RD population could be in hundreds of millions; however, each disease may represent hundreds or thousands of patients, worldwide. Due to the lack of profitability, pharmaceutical companies stopped developing drugs to treat rare diseases patients. In 1983, to provide incentives to pharma companies, the Orphan Drug Act (ODA) was established in the USA [4]. ODA became a guiding light for many countries in the world. By the end of 2017, more than 450 products, for 668 orphan indications were approved by the FDA [5]; while before the ODA, the orphan drugs were fewer than ten. These orphan drugs are not available in India due to import restrictions, unaffordable prices, etc. Further, there is neither such ODA nor any other provisions available to patients in India. As a result, 80-90 million RD patients are suffering.
In India, awareness of RDs lacks, not only the general population but health care professionals, as well as policymakers. Expertise and specialized institutions are scarce. Significant barriers to access diagnostics and care facilities exist. Documentation of rare diseases within the healthcare system is insufficient. Due to the lack of a centralized disease-specific registry and data collection system, there is no valid information on the epidemiology of rare diseases in the country.
Indian Organization for Rare Diseases (IORD) [6] is an umbrella patient organization representing all rare diseases and rare disease patients in India. Its mission [7] is to raise awareness, advocate public policy, encourage pharma companies [8] to develop drugs and biotech companies, academic institutions to developing diagnostic tools [9]. During the last fourteen years of her service to the RD community in India, IORD recognizes that RD not only affects the patients but also affects the entire community (remaining 92% of non-orphan) - physically, emotionally, and financially.
In India, often a common question arises among policymakers regarding the number of rare diseases and the number of patients suffering from RDs. As India does not have the definition of rare diseases, it is hard to estimate accurately the number of patients suffering from rare diseases. Therefore, it is challenging for policymakers to draft the health care policy that adequately addresses the needs of patients, provides incentives to the pharma industry to develop drugs [10], develop tertiary infrastructure to treat RD patients. In 2017 the Government of India released a policy document [11], and while implementation, it faced hurdles [12].
Over the years, several surveys were conducted in developed countries. However, they are often focused on specific issues such as access to orphan drugs [13], the role of RD patient groups in research [14], challenges faced by patients [15], issues related to rare disease caregivers [16], the social media platform to contact patients [17], patients' survey [18]; balancing care and life [19]. Rare Barometer Voices [20] conducts surveys very frequently on a variety of topics.
IORD initiated a 'first-of-its-kind' survey to obtain a systematic and baseline knowledge of RD in the country. No such survey has ever been conducted by any government or any agency in any country. It is not practical or doable to survey 1.3 billion people in a country with multiple states, languages, economic and social inequalities, and a host of other reasons. We expected a well-represented sample survey to give meaningful information, relatively quickly and inexpensively. Further, the purpose of this survey was to provide a snapshot, the baseline understanding of rare diseases in the country. The information from this survey will be aggregated and compiled for a report and is freely available to anyone. It was expected that the study would give impetus to the national conversation on care and support for rare diseases' families, and provide greater awareness of how RDs impact communities.
There are significant resources for educational materials available to most people in developed nations. However, millions of individuals and families in the developing nations around the world do not have access to most of these resources except to those who can access these services [21] through the internet. The target audience for this survey were those who access to the World Wide Web and would understand the purpose.
Due to the large rural population, low literacy, the survey was limited to the population who could be reached through the internet. The sample survey was obtained from a subset of the population with the criteria arbitrarily set as people with above-average literacy such as college degree, which could easily be reached and expected to understand the purpose. For a broader survey, staff, funding, and time are the limitations. The survey was conducted by selecting units from a population, which comprised medical, and non-medical fraternity that could be meaningfully thought of as defining a population to be studied. The participants should be residing in India, or non-resident Indians (NRIs) [22] living in the United States. Non-sampling errors (excluding certain population), may have reduced precision in our estimates. It is difficult to quantify coverage error without special studies of the un-sampled portion of the population. Further, practical issues arose because of the complexities of gathering information on many real issues, for example, knowledge of the known rare diseases, diagnosis, and treatments, etc. The survey instrument involves a questionnaire that provides a script for a standard set of questions and response options (Supplementary Material and Survey Questionnaire).
The questionnaire was designed to collect information specific to RD and OD in such a way that the answers are either 'yes' or 'no' type. 'Multiple-choice questions' and some others require open-ended responses - what orphan diseases and orphan drugs are, number of people suffering from RD, causes of RD, cost of medicine, affected age group, the time taken to diagnosis, which group of people affect most, etc. A separate section was included to the practicing physicians who are asked specific questions, - e.g., how often RD patients are seen in their practice and their available resources for diagnosis and treatment, etc. At the end of the survey, the respondents have the opportunity to leave a comment. The survey responses are compiled in (Table 1, Table 2, Table 3, Table 4 and Table 5).
Table 1: Male and female Respondents. View Table 1
Table 2: Age groups, medical and nonmedical fraternity respondents. View Table 2
Table 3: Awareness levels of respondents on RD & OD. View Table 3
Table 4: Awareness/non-availability of resources among healthcare professional. View Table 4
Table 5: Number of people identified rare diseases when given along with common and neglected diseases. View Table 5
The average time the respondents would take to complete the survey is expected to be twelve minutes. The survey takers were advised not to refer to Google or other materials and not to consult others for answers; as such attempts would skew the results and defeat the purpose. The survey takers are informed that the objectives of this survey are to determine baseline knowledge of RD and OD in India.
The Survey Questionnaire was sent randomly to people in India, and NRIs in the USA via their e-mails. The participants come from major cosmopolitan cities in India and are limited to urban areas [23]. The survey takers come from randomly selected States in India and some Western, Eastern, and Midwestern States in the United States. The emails of survey takers are obtained from the public, the professional associations, regional non-governmental associations, pharmacy, medical, nursing educational institutes/schools, practicing physicians, industry personnel. The minimum qualification of participants is a college degree in any area of disciplines such as arts, science, and professional degrees in information technology, medical, pharmacy, and nursing, etc. The data was collected between the periods of December 2017 to October 2018.
A Survey Questionnaire was e-mailed to 6005 people, and 599 responses were received. The ratio of females (248) to male (351) was 0.69 [24] (Table 1). Among the respondents, 80% were from India, and the rest of them were from abroad. Responses were evenly spread across all age groups. The responses of the age groups, > 50, 18-20, and 29-30 are the lowest at 8%, 7.7%, and 7.6% respectively and the responses were highest with age groups, 41-40, 21-23, were 21.2% and 18.6% respectively. The next age group 24-28 was 14.9%. The nonmedical respondents had the highest participation at 67.7%, whereas the practicing physicians [25] was at 5.7% (Table 2). 32.3% of respondents of the survey were with medical, pharmacy, or nursing background with a minimum of an undergraduate degree; among them, 36.3% of people had one or more scientific publications, whereas 58.9% of respondents had a membership in professional societies. 70% of respondents had not participated in any voluntary organizations. 30% of respondents indicated that their institutions did not offer rare disease awareness programs. 23.7%, 42.6% of respondents never heard the term "rare diseases" and "orphan drugs", respectively. 17.6% of respondents thought poor countries and 8% of respondents felt that the rich countries suffer most while 74.4% felt that RDs do not discriminate rich or poor (Table 3). 37.6% of respondents did not know the definition of a rare disease. Similarly, 48.4%, 57.2% respondents unaware of the number of RDs and patient population in the country respectively, while 66% of respondents did not know the number of commercially available ODs. 41.6% of respondents think the common cause of rare diseases was genetic, but 20.3% of people were unaware of the cause for rare diseases. Only 14% of respondents think that rare diseases occur by birth, while 48.5% thought they occur at any age. However, 19.3% of respondents did not know the age of onset. Some respondents think RDs effect India more than China, but 67.7% thought rare diseases affect all countries. An approximate time took to diagnosis a rare disease was equally spread from one week to five years. 36.2% of respondents thought that rare diseases affect only 1-2% of the population, while 30.3% of people did not know the affected population. Practicing medical professionals included were medical students, pharmacy, nursing, and practicing doctors. They represented 47.1% (247 respondents) in the survey. From these healthcare professionals, 42.8% had not seen any rare disease patients. Thirteen rare diseases from the National Organization for Rare Disorders (NORD) website [26] and twelve common diseases and four tropical diseases were listed to identify the rare diseases (Table 5). 77.6% had no resources to diagnose, while 80.1% had no resources to treat rare disease patients, and 76% had no continuing education programs in their institutions (Table 4) (Supplementary Material: Graphic Representation of the responses).
All the information from this survey is completely anonymous and does not collect personal or identifiable information about the respondents or institutions, and there is little or no potential for invasion of privacy or breach of confidentiality. This information is essential to assure the survey takers voluntarily complete the study. The respondents were informed before taking the survey that the information derived from the study would be reported as a public report or a journal publication. Also, the study may be made available to India's Ministry of Health and Family Welfare, and others who create programs and services for parents and caregivers. It is expected that the sample study will help to move the national conversation on care and support for rare disease patients and families and provide greater awareness of how rare diseases affect communities. Health budgets are insufficient to provide care for all. Allocating resources to treat a person with a rare and expensive disorder over a person with a more common, less expensive disorder is a problem for cautious policymakers who are concerned about justice and fairness in allocating their community health resources. Therefore, establishing the baseline knowledge of RDs in the country is essential.
Among all the respondents, one third have one or more scientific publications and have professional society membership. This number of professionals is very close to the respondents who indicated that they are medical professionals (students, physicians, etc.). This fact is considered as an indication that a subset of the respondents has some understanding of RD.
Among the respondents, the oldest and youngest generation appears to be less familiar than other age groups. NRIs who are generally expected to have a higher standard of living appears to have the same awareness as people living in less privileged places in India. The nonmedical respondents have the highest participation, whereas practicing physicians are fewer. This information is in line with the ratio of medical and non-medical participants. These results are in parallel to the familiarity of the awareness of RD among the respondents who are health care professionals and those participants with voluntary and, professional membership and publications. One-fourth of the participants have not heard the terminology of RD and OD. It may be that there are no programs in their medical and other educational curricula. Again, it appears that there is a correlation between the knowledge of rare diseases and awareness programs.
The definition of rare diseases varies from country to country. The three-fourths of respondents are not familiar with a definition for RD. The knowledge of the cause of rare diseases is variable; one-fourth people have no idea what causes the RD. More than half of the respondents are unaware of the number of RD and the number of people suffering from RD. Half of the people think the RD can occur at any age. The time to diagnose the RD is evenly spread from one week to five years.
The question relating to rare diseases population in the country, half of the respondents indicated that they either do not know the definition for a rare disease or do not know what Rare Diseases are. Similarly, half of the respondents do not know the number of rare diseases and the number of orphan drugs. Since 1983, many global companies started developing orphan drugs after the Orphan Drug Act implementation. There is none at this time in India, although in 2017, the Drugs & Cosmetic Act 1945 has been amended [10] to include "rare diseases and orphan drugs". Half of the practicing medical professional group has not come across the term RD, and three-fourths of them have neither facility to diagnose nor treatments for the rare diseases (Table 4). From the list containing rare diseases, common diseases, and neglected diseases provided to the survey takers, only 50% of people have correctly identified six of thirteen RDs (Table 5). Unlike the USA and other developed countries, there are not many patient organizations in India [27], and respondents indicated that their institutions do not offer rare disease awareness programs. This response most likely comes from medical professionals.
Out of 599 respondents, only 243 have some connection to healthcare. The lack of information on rare diseases or general awareness of its issues is not unique to India. The world's most populous country, China has the world's largest number of rare disease. However, these countries have no system of registering cases of rare diseases. Therefore, there is very little documented information on the epidemiology of those diseases in the most populous countries. Although developed countries have well-established policies, they are now recognizing the importance of developing countries [28]. India's exposure to rare diseases is limited, particularly international exposure. Policymakers in India are catching up to determine the status of rare diseases in the country. Patients and patient organizations do not have a voice. Therefore, they are not part of discussions for creating National Health Policy or in the review process of existing policy documents [29].
At the end of the survey, the respondents were asked to leave comments or suggestions. A good majority of the respondents have opted for awareness programs as the primary focus. It is said awareness brings cures, help policymakers to realize the importance of RD in the healthcare system. Many felt the awareness of RD is the first step for everything-for the definition, health care policy, diagnosis, treatment. Through education at all levels - college or professional education - the awareness could be brought through the public health. Funds could be collected for research from various sources-investors, companies, government, and philanthropy- suggesting private-public-partnerships is vital. The government should support the discovery and development of Orphan drugs [30]. Many respondents suggested including rare diseases in public policy as a priority issue. Interestingly some suggested involving the United Nations to make RD a global priority [31]. Diagnostics has taken the next priority after awareness programs. New programs are to be created to include rare diseases in the medical college curriculum. Many suggested that having seminars, workshops, and RD health camps in rural areas are important. Practicing physicians expressed concerns about not having practical experience. Medical and paramedical students expressed that they did not have much information about the rare disease. Some others, suggested incorporating distinguished lecture series in the university and a chapter in medical textbooks is mandatory. Some respondents suggested to creating databases on RD and OD. It is suggested to have specialized hospitals.
Given the current situation of rare diseases in India, knowing where India stands in this regard is an important first step for drafting the rare diseases policy and providing incentives to orphan drug development to improving the quality of patients and host of other things. The purpose of this sample survey is accomplished by obtaining baseline knowledge of rare diseases in the country. The survey is given to people with a minimum of a college education and people living in metropolitan cities and to Indian people living in the USA. The survey obtained information on issues such as definition, population, orphan drugs, education, and policies. The survey categorized two sets of people; one with limited knowledge of health, and others have no connection to health. The results indicate the awareness in all categories falls short. It is found that more than 70% of people in India do not have basic knowledge of rare diseases. Sixty percent of people with little or no formal education live in rural areas. Therefore, the number of people with no knowledge of rare diseases would increase substantially if the rural population is included in the survey.
This survey suggests that The government of India to drafting an RD policy document, defining the RD, and creating the Orphan Drug Act should give careful consideration should. The sample survey results are consistent with the expectation that India needs to do more to raise the awareness of rare diseases, for example, include the RD in the 2021 census.
We thank Mary Dunkle, National Organization for Rare Disorders for her suggestions, encouragement from the conception to the finishing of this paper. I sincerely acknowledge William Toscano, and George Maldonado, School of Public Health for their advice, Robert Straka, Experimental Clinical Pharmacology, for reading the manuscript and Danielle Carrigan, for technical assistance, college f pharmacy, University of Minnesota.
The authors have no conflict of financial interest.