Background: Wide practice variation and weak guidelines exist when considering enteral feeding options and the decision to proceed with gastric or postpyloric enteral feeding in critically ill infants and children. The purpose of this pilot study is to explore provider decision making in early feeding of critically ill pediatric patients, review the indications for choice of enteral feeding access, and qualitatively explore the role of institutional culture in medical decision making.
Methods: In-person structured interviews of multi-level providers were conducted over a two-week period in PICU, NICU, and pediatric cardiac intensive care unit at our facility. A survey was developed to evaluate practitioner demographics as well as decision-making for feeding access in mock scenarios. Descriptive analysis was used for demographics and quantitative responses. Interview transcripts were analyzed using qualitative content analysis to identify common themes and variance in decision making.
Results: Providers selected gastric feeding for most of the mock scenarios, a median of 7.4 times for 10 scenarios [IQR 6-9]. At least 1/3 of respondents selected postpyloric feeding for patients with neurologic impairment, hypotonia, aspiration pneumonia, and severe burns. 85% (n = 22) felt institutional culture plays a role in their decision making. 52% (n = 14) felt that postpyloric tubes result in more complications.
Conclusion: Initial feeding tube decisions vary among providers, and many feel institutional culture and personal experience play a role in these decisions. Stronger feeding guidelines may help to decrease variability in enteral access choice.
Enteral nutrition, Practice guideline, Pediatrics, Critical care, Institutional practice
Early nutrition is important for critically ill pediatric patients and has been shown to improve mortality, reduce infection, increase caloric intake, and improve healing [1-3]. There are strong recommendations for enteral nutrition over parenteral nutrition as the preferred method of delivering nutrients to critically ill pediatric patients, but there is currently no empirical consensus on whether gastric or postpyloric feeds are best for critically ill pediatric patients [1].
While implementation of feeding protocols can help earlier initiation of enteral feeding, current guidelines in regard to feeding the stomach or small intestine have low quality of evidence and weak GRADE recommendations [1,4-7]. General consensus is that feeding the stomach first is preferred as it's more physiologic, especially when bolus feeds are used, and that postpyloric feeds should be used in situations where initial gastric feeding has failed [1,2,8-10]. However, postpyloric feeds may be used empirically to prevent complications of gastric feeds in patients with reflux and vomiting, decreased mental status, poor gut motility after surgery, or hemodynamic instability [2,11]. Institutional practice varies widely and sometimes postpyloric feeds are initiated in critically ill neonates and children as the first method of tube feeding in order to reduce the risk of aspiration [6,12,13].
Without strong, evidence-based guidelines, decisions are left up to individual institutions and providers, and lack of evidence-based protocols leads to varied practice and preventable complications [6,7]. Even when guidelines exist, individual provider experiences and patient circumstance can influence medical decisions. An understanding of provider reasoning is necessary to create effective protocols [14,15]. The purpose of this study is to explore provider decision making in early feeding of critically ill pediatric patients, review the indications for choice of enteral feeding access, and qualitatively explore the role of institutional culture in medical decision making. The goal is to utilize this information for the creation of locally adapted patient care guidelines.
Following IRB approval, a four-part survey was developed for semi-structured in-person interviews. The first part assessed provider demographics (Table 1). Then, providers were given 10 hypothetical scenarios of patients being considered for tube feeding and were asked if they would start gastric or postpyloric tube feeds (Table 2). Next, providers were asked to rate how much they agreed or disagreed with twelve statements about enteric feeding methods on a five-point Likert scale (Table 3). Finally, providers were given five free-response questions in which they were asked to elaborate on their decision making and opinions on institutional culture (Table 4). A mixed-methods approach was taken so that the qualitative portion of the study could fill in gaps where our quantitative portion may not have given the participants the chance to expand on their decision making.
Table 1: Participant demographics. View Table 1
Table 2: Scenario Responses. Modes of initial feeding participants chose for mock patient scenarios. View Table 2
Table 3: Participant responses of how much they agreed or disagreed with statements on a Likert scale of 1-5. View Table 3
Table 4: Free response questions. View Table 4
The survey was first tested on 2 attending surgeons, 3 nurse practitioners, and 1 physician assistant in the pediatric surgery division using cognitive interviewing [16,17]. None of these providers were included in the actual survey. After each question, the providers were asked how they understood the language in each question, if the topics fit the context of the study, and if they had any suggestions to make the survey clearer. After each cognitive interview, the provider's suggestions were taken into consideration and discussed among the researchers. If the suggestions were consistent with the purpose of the survey, the survey was revised. Providers made suggestions such as providing a visual Likert scale and gave examples of follow-up questions that might be helpful to ask the intensive care unit providers, which were implemented in the final survey. The survey was edited between each cognitive interview, so most suggestions were tested multiple times.
Interviews were conducted over a two-week period in the pediatric intensive care unit (PICU), neonatal intensive care unit (NICU), and pediatric cardiac intensive care unit (PCICU) of a tertiary children's hospital. Convenience sampling of resident and fellow physicians, advanced practice providers, and attending physicians was conducted to ensure a diverse sample of participants, with a goal of at least 5 participants from each unit and level of training deemed to be sufficient representation. Following informed consent, semi-structured interviews were conducted by a single trained interviewer (AM) and audio recorded. The format of semi-structured interviews allowed for the interviewer to follow-up hesitancy or uncertainty in respondent answers with open-ended questions, prompting respondents to explain their decision-making rationale. By default, respondents were prompted to elaborate on a minimum of two questions in each part of the survey. Interviews were then transcribed into a RedCap database and edited for anonymity.
Quantitative data from discrete interview questions was analyzed using descriptive statistics in SAS 9.4 (SAS Institute, Inc, Cary, NC). Conventional qualitative content analysis was performed by two independent researchers (AM, KE) to identify common themes from the survey responses [18]. Inductive reasoning was used to identify common concepts and phrases. The concepts and phrases were then categorized into themes by how they related to each other and to specific survey questions. Divergence in theme analysis by the researchers was decided by the senior author (RP).
A total of 27 interviews were conducted, 26% in the NICU (n = 7), 48% in the PICU (n = 13) and 26% in the PCICU (n = 7). Respondents included 10 attending physicians, 7 fellows, 5 residents, and 5 advance practice providers (Table 1). 67% (n = 18) of participants completed their highest level of training at the study institution, and 67% had practiced outside of the study institution at some point in their careers. 81% (n = 22) of participants had spent at least 2 years working at the study institution at the time of their interview.
Providers selected gastric feeding as the initial feeding for most scenarios, a median of 7.4 times for 10 scenarios [IQR 6-9]. There was no statistically significant difference in the frequency providers selected gastric feeding based on practice location (p = 0.53), provider type (0.20), or increased time at the institution (p = 0.20). However, scenarios where at least 1/3 of respondents selected postpyloric feeding included patients with neurologic impairment, hypotonia, ductal-dependent congenital heart disease, and severe burns (Table 2). Participants with more than 5 years (n = 9) at the institution were less likely to choose postpyloric compared to those with less 5 years (p = 0.04). Additionally, attending physicians were less likely to choose postpyloric feeds, although this was not statistically significant (n = 0.06). Due to the small sample sizes, further multivariate analysis was not possible, and significant bias is possible in presenting these results utilizing quantitative analysis.
The scenario with the most consensus among providers was "a premature infant with low birthweight". 96% of providers (n = 26) chose to feed this patient with gastric feeds, and one participant answered, "could not determine". The scenario with the least consensus was "A 12-year-old with cerebral palsy admitted with aspiration pneumonia". 48% (n = 13) of participants chose gastric feeds for this patient, 44% (n = 12) chose postpyloric feeds, and 7.4% (n = 2) could not determine. When asked to elaborate on decision-making, participants primarily wanted to know the patient's respiratory status, swallow study results, and prior history of aspiration pneumonia.
In response to a series of statements on both feeding methods, 26% of participants (n = 7) agreed that patients with lung disease and reflux symptoms should not have gastric feeds (Table 3). 48% of participants (n = 13) disagreed that gastric tubes are more likely to lead to aspirations than postpyloric tubes in critically ill patients, and only 33% (n = 9) agreed. However, in free response portions of the interview many participants cited aspiration risk as a main factor for starting a patient on postpyloric feeds.
Views also varied on how different surgeries should be considered in feeding decisions. An equal number of participants agreed (44%, n = 12) and disagreed (44%, n = 12) that a Nissen fundoplication prevents aspiration. Only 7% of participants (n = 2) agreed that patients post-sternotomy should receive postpyloric feeds to prevent aspiration. Specifically, several participants indicated that heart surgeries that involve arch manipulation or a ductal dependent disease require postpyloric feeds while one participant thought that all post-sternotomy patients received postpyloric feeds.
The statement that had the most consensus among participants, was that many patients initially fed with nasogastric tubes often end up needing postpyloric tubes; 85% (n = 23) disagreed. Participants most strongly disagreed with the statement that neonates who start with postpyloric feeds have a shorter length of stay than those on gastric feeds. This statement had the second highest consensus, with 74% (n = 20) disagreeing overall. The two statements that were agreed with the most were that use of postpyloric tubes as the initial feeding method results in higher costs (59% (n = 16) agreed), and that practice guidelines make it clear how to initiate tube feeds in your unit. However, regarding guidelines, only 15 participants (55%) agreed.
Themes and sample patient responses are highlighted in Table 5.
Table 5: Themes and quotes. View Table 5
Providers' decisions largely depend on specific patient circumstance: When asked to choose between gastric and postpyloric feeding for sample patient scenarios, many participants felt the scenarios were too vague and wanted more information about how critically ill the patient was. When asked to elaborate on their decisions, most participants (n = 18) communicated that they prefer to start gastric feeds but must take the patient's specific clinical circumstance into consideration. Many said they would consider what respiratory support the patient was on, their feeding history, their reflux and emesis status, the results of a swallow study and vocal cord evaluation, origin of previous aspirations, and surgical needs (Figure 1). Other considerations included hemodynamic stability and recommendations from speech and language and occupational therapists. Additionally, as postpyloric tubes are placed at our institution by a special nursing team utilizing the Cortrak 2 Enteral Access System (Avanos Medical, Alpharetta, Georgia) for placement, providers expressed time of day and availability of this team as a factor in decision-making. Only 4 participants mentioned a unit protocol in their free responses, and when participants believed a patient was more critically ill, they tended to have a bias for postpyloric feeding.
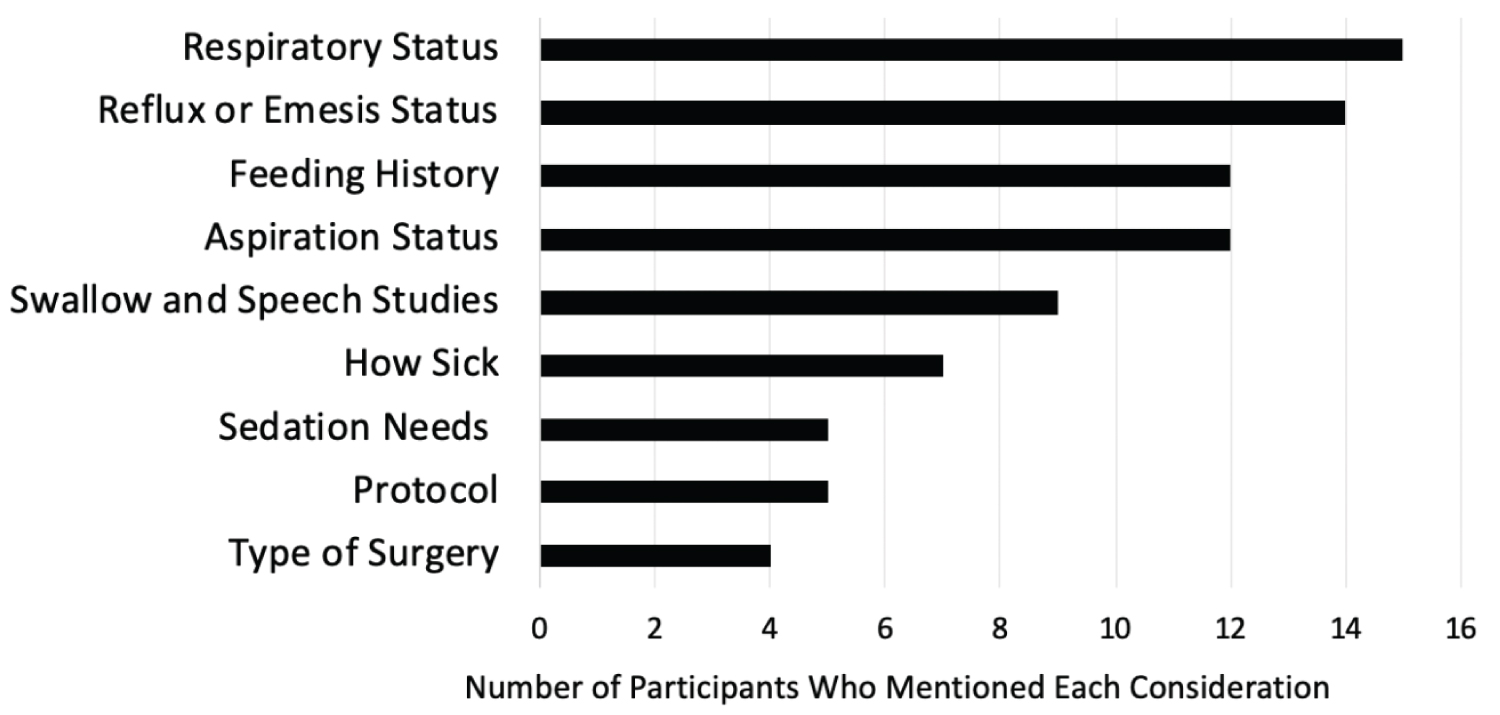 Figure 1: Most common considerations for enteral feeding decisions. View Figure 1
Figure 1: Most common considerations for enteral feeding decisions. View Figure 1
Providers prefer to start patients on gastric feeds: Many participants stated that gastric feeds should be started first because they are more physiologic, and if not tolerated, then postpyloric feeds should be considered as a second option. 48% (n = 13) disagreed with the statement that gastric tubes are more likely to lead to aspirations than postpyloric tubes in critically ill patients, while multiple participants stated that evidence does not show postpyloric feeding reduces aspirations. Two participants stated that most aspirations are from the mouth, so it is safe to start with gastric feeds. Other reasons stated for initiating gastric feeds first included that the patient is stable, not "on a lot of respiratory support", or a Nissen fundoplication was done that prevents aspiration. Multiple participants stated that bolus gastric feeds are better because they are more comfortable for the patient and their families compared to continuous postpyloric feeds.
There are some reasons to start postpyloric feeds first: Participants stated that suspicion or documentation of reflux, vomiting, and aspiration were all reasons to start postpyloric feeds first. Multiple participants also stated postpyloric feeds should be given if the patient is hypotonic, has poor coordination, on noninvasive high-pressure ventilation, not neurologically intact and wearing a breathing mask, or there is suspicion of impending respiratory failure. Other commonly stated reasons for empiric postpyloric feed included if patients would need frequent sedations, such as burn patients, or if they had arch manipulation during surgery.
Institutional culture plays a role in decision making: 82% of respondents (n = 22) felt that institutional culture plays a role in their decision making. Reasons cited included lack of evidence, bias from personal experiences, and trainees basing decisions off superiors' past decisions. Many participants also described different trends in decision making depending on the unit or institution in which they worked. Out of the 5 participants who felt that institutional culture does not play a role in their decision making, 4 were attendings, 1 was an advanced practice provider, and all units were represented among them. 2 of these 5 participants stated that while institutional culture did not affect their own decision making, they believed it affected other providers' decisions.
More research needs to be done on this topic and guidelines would be useful: Most participants (96%, n = 26) supported ongoing exploration of feeding tube decisions, with 7 participants specifically stating that more research is needed to determine the safety and efficacy of each feeding method for critically ill pediatric patients, and 4 participants stating their desire for more evidence-based guidelines and protocols.
Variable viewpoints exist surrounding complication rates of each method: 48% (n = 13) of participants felt that use of postpyloric tubes as the initial feeding method results in more complications. Personal experiences with situations resulting in a bowel perforation created a strong bias on qualitative interviews. 44% (n = 12) of participants felt that both feeding methods have similar rates of complications and that outcomes depend on the clinical scenario. One participant said gastric feeds cause more complications.
Complications described by participants surrounding postpyloric tubes included bowel perforation, tube displacement, tube clogging, extra imaging, patient discomfort from continuous feeds, inadequate growth, prolonged hospital stay, and difficulty with wound healing in burn patients secondary to diarrhea. Gastric feed complications that were mentioned included reflux, emesis, aspiration, gastric erosions, and stomach perforation. Participants who did not believe that one method has more complications over the other communicated that both methods have their pros and cons, and severe complications are rare.
Feeding with a gastric tube initially may result in a better feeding situation on discharge: In response to the question of which initial feeding method results in the best feeding situation at discharge, 77% (n = 21) of participants chose gastric feeds, none chose postpyloric, 26% (n = 7) said it would ultimately depend on the patient, and 1 participant said initial feeding method would not make a difference on feeding situation at discharge.
Most participants stated that initially feeding with a gastric tube results in better feeding situation at discharge because it is more physiologic and can be done in bolus feeds, allowing an easier transition to feeding by mouth by the time of discharge.
Given the lack of empirical consensus on which critically ill pediatric patients may benefit from empiric postpyloric feeds, guidelines at our institution are not defined, and decisions are consequently left to individual units and providers. This study aimed to explore how pediatric providers at our institution decide which feeding tube method to use, their overall perceptions on each method, and the extent to which they believe institutional culture plays a role in these decisions. The study was conducted by a surgical team who suspected variance in practice. While not often involved in initial decision-making for non-surgical patients, they are part of the multidisciplinary team in eventual feeding access or management of complications. The results showed that perspectives on each feeding method and when to use them vary widely among providers at our institution, and that many providers think more inter-unit conversations and evidence-based guidelines would help make decisions consistent. This has significant implications for decreasing variability in patient care, which has ultimately been linked to improved outcomes, improved efficiency, and decreased healthcare costs [6,7,19,20].
Many participants communicated that gastric feeds should be tried before postpyloric feeds for most patients, and this is consistent with available feeding guidelines [1]. The North American Society For Pediatric Gastroenterology, Hepatology & Nutrition (NASPGHAN) and American Society for Parenteral and Enteral Nutrition (ASPEN) suggest that the gastric route be the preferred site of enteral feeding except in cases of gastric feeding intolerance and increased aspiration risk [1,21]. Reasons for increased aspiration risk may be a past history of proven reflux or aspiration, positive pressure ventilation, intubation, neurologic deficits, or recent heart surgery, however sometimes it is patient specific and hard to define [2,22]. In our study, over 85% of participants selected gastric feeds for the patient with the most severe respiratory problems, yet respiratory status was one of the main concerns of providers when they described their decision-making process. Interestingly, while aspiration risk is the most prevalent contraindication for gastric feeds, there is no consistent evidence for reduction of aspiration with postpyloric feeds [11,23,24]. Also, an equal number of participants agreed and disagreed that Nissen fundoplication prevents aspiration, when studies have shown that Nissen fundoplication is not effective in reducing aspiration rates among critically ill patients [25,26].
The most consensus surrounds feeding preterm and low birth weight infants [8,11]. A meta-analysis of 9 trials and 359 preterm infants found that postpyloric access leads to more gastrointestinal disturbance and did not find a significant difference in feeding tolerance, aspiration risk, or in hospital growth rates between each method [11]. Our survey mirrored this consensus as the two scenarios with the most votes for gastric feeding being the premature infant with low birthweight (93%).
Neonates and children with congenital heart disease are unique in that many of them receive surgery that can injure their recurrent laryngeal nerve and therefore swallowing abilities, are often given sedatives and muscle relaxants which may increase risk of aspiration, are a heavily ventilated population, and often receive clinical care that requires cessation of gastric feeds. Postpyloric feeding is safe in this population and allows delivery of nutrition with fewer interruptions, and participants in our survey described a significant prevalence of postpyloric feeding of these patients at our institution [10]. Yet, some cardiac units still use gastric bolus feeds as the most common method [10,12]. A protocol for postoperative feeding of patients with hypoplastic left heart syndrome that starts with gastric feeds and only gives postpyloric feeds to patients who have a history of feeding intolerance or vomiting was found to be safe and effective at advancing feeds [27]. The Pediatric Cardiac Intensive Care Society has called for more clinical research and quality improvement to determine best-practice feeding guidelines. Institutions that implemented more strict feeding guidelines for their pediatric cardiac patients had better outcomes, and wide practice variation currently exists [5,12,13].
Regarding complications, participants found fault with both feeding methods. A single-center prospective study found that mechanical issues were responsible for 43% of enteral nutrition interruptions for their patients, including tube malposition, obstruction, and placement failure, and postpyloric tube malfunction is especially prevalent [22,28]. Some studies found that postpyloric feeds help participants reach feeding goals more quickly and have reduced length of stay, whereas others found that gastric feeds help patients shorten the time needed to reach nutritional goals [24,29]. As mentioned by some of our participants, postpyloric tubes require more radiographic studies than gastric tubes and may result in higher costs [1,24]. About 44% (n = 12) participants believed neither method has more complications than the other, and a prospective study found no difference in feeding intolerance, micro aspiration, or tube displacement between postpyloric and gastric feeds [23]. References to bowel perforation as a result of postpyloric feeding were numerous among our survey responses and created a strong bias for gastric feeding for some providers.
Given the lack of empirical consensus on the topic, it was determined that semi-structured interviews with open-ended questions would be best to evaluate provider reasoning. We used conventional content analysis, which is generally used when trying to evaluate something for which research is limited and is best used to analyze open ended and probing questions [18].
Due to convenience sampling, not every provider in each unit was surveyed, implicating a selection bias of available providers. Additionally, small sample sizes and the inherent variability in the semi-structured interviews and provider selection make the quantitative analysis subject to Type 2 error and unreliable in drawing conclusions on variation between units or provider type. Because our survey was framed around a conventional content analysis model, participants were asked open-ended questions, and were not always prompted for the same additional information in respect to their answers. However, given that this was a pilot study, we still gained important insight into a variety of providers' decision-making processes.
Some participants felt that the scenarios for which they were asked to make decisions were too vague; however, this was intentional. In-person interviews were conducted so that the interviewer could ask the participant what additional information they may need to make their decision. This allowed the researchers to gain a sense of what was important to participants and identify information that could be important to include in future protocols. An important limitation of our survey was that opinions on certain aspects of feeding methods or decision making were not obtained by every participant and this may explain some variation in responses to different parts of the survey.
Our survey showed that providers at our institution have a variety of perspectives regarding feeding critically ill patients into the stomach or small intestine, and institutional culture and provider experience plays a role in most participants' decision-making process. This qualitative study is part of a larger project to retrospectively evaluate outcomes, complications, and cost of initial gastric versus postpyloric feeding at our institution to develop contextually appropriate evidence-based feeding guidelines. Our results illuminate some of the reasoning behind feeding decisions and hopefully can initiate conversations among providers and units who have yet to examine their practice. More research studies on clinical outcomes of gastric and postpyloric feeding in critically ill children are needed to inform stronger guidelines and protocols on when to use each method, as well as provide more insight into the costs and complications of each method. Our study also highlights that it's important to consider the non-evidence-based factors that go into these decisions to create effective protocols and positive change.
I would like to thank Rachel Nettle, ARNP, Erin Murray, ARNP, Ashleigh N Markowitz, PA, and Lauren Indelicato, ARNP, all providers in the Division of Pediatric Surgery at our institution, for participating in cognitive interviews and providing feedback during survey development.
This study was previously presented as an abstract and QuickShot presentation at the 15th Annual Academic Surgical Congress. No financial support was used for this study.
All authors attest that they meet the current ICMJE criteria for Authorship.