Background: Malaria is a parasitic, life-threatening, vector-borne disease that is a significant public health problem in sub-Saharan Africa. The disorganization of health systems has characterized the Covid-19 pandemic period. This study aimed to assess the impact of these changes on adolescent malaria.
Methods: We conducted a longitudinal study in the 2021 in the pediatric ward. We enrolled all patients treated for malaria based on a positive thick smear and rapid diagnostic test for malaria. We noted the patient's age, delay to consultation, signs at admission, haemogram test results, parasite count, and duration of stay.
Results: Of 1734 inpatients, 689 (39.7% (95% CI [37.4%-42%]) had malaria, including 128 adolescents. We rated 417 (24.3%; 95% CI [22.3%-26.3%]) cases of severe malaria, including 94 (73.4%; 95% IC [65.8-81.1%]) adolescents. The median delay to adolescent consultation was 2 days vs. 3 days for children p = 0.004. The admission signs were fever (98%), prostration (38%) and pallor (32%). The median length of hospital stay for adolescents was 3 days vs. 4 days for children (p = 0.0263). Adolescence was associated with more severe forms (OR = 2.04 95% CI [1.33-3.13]) and more cerebral forms (OR = 3.8 95% CI [2.6-5.6]).
Conclusion: The post-COVID-19 era was characterized by a higher incidence of malaria and its severe forms in adolescents, primarily cerebral malaria. Due to potential sequelae, they need a follow-up to estimate the actual aftermath of the pandemic on the pediatric population.
Adolescent, Cerebral malaria, Covid-19, Gabon
Malaria is a parasitic, life-threatening, vector-borne disease that is a significant public health problem in tropical countries, particularly Sub-Saharan Africa. It is both preventable and curable. In its latest report of 2023, the WHO estimated that by 2022, there were 249 million cases of malaria worldwide, 16 million more than the 233 million recorded before the covid-19 pandemic in 2019 [1]. The number of deaths attributable to malaria has risen to 608,000, during the COVID-19 crisis. The WHO African Region bears a significant and disproportionate share of the global malaria burden, with 94% of malaria cases and 95% of malaria deaths recorded in this region [1].
Children under the age of 5 years account for an estimated 80% of all malaria deaths in the region. Together with pregnant women, they are the main targets of action for combating malaria [1]. Malaria in Gabon is hyperendemic, with persistent transmission favoured by the country's equatorial climate, and Plasmodium falciparum is responsible for 94% to 99% of malaria attacks [2,3]. In Gabon, the government's implementation of national malaria control measures in 2002 led to a decrease in the prevalence of infection among children, from 31.2% to 18.3% between 2000 and 2008 in Libreville [4]. We observed a return to an increase in the prevalence of malaria as a cause of hospitalization among children since 2013 [5].
The COVID-19 crisis has negatively impacted national health systems, including managing diseases such as malaria and non-communicable diseases [6]. Healthcare capacity has gradually recovered to its potential, from 50 to 90% of disruption in 2020 to approximatively 30% of efficacy in the first quarter of 2021. There is little data on the prevalence of malaria among adolescents. Some studies have claimed that adolescents are also at high risk of malaria-related infections and complications [7].
Given the epidemiological data on the recovery from malaria in our context, what was the situation of adolescents concerning malaria in the period following the COVID-19 pandemic, especially the forms seen in hospitals? The objectives of our study were to assess the frequency of complicated forms of malaria in hospitalized adolescents, identify the most common severe clinical form of malaria in adolescents in our department and determine the factors associated with severe malaria in adolescents.
We conducted a prospective, longitudinal and analytical study from January to December 2021 in the paediatric department of the University Hospital of Libreville. This department is the largest paediatric ward in Gabon, with a hospital capacity of 60 beds, a capacity reduction of 40 beds in the post-COVID-19 period. The survey concerned children of all sexes hospitalized in this department aged 1 month to 17 years.
The inclusion criterias were as follows: 1 month to 17-years-old, a positive thick smear or a positive malaria rapid diagnostic test (RDT), a parasite count, and at least one malaria severity criterion defined by the WHO. The exclusion criteria were any hospitalized child with a thick drop of malaria and RDT negative; no parasites count. The criteria for withdrawal from the study were the finding of bacterial, viral, or fungal co-infection, including bacterial meningitis or encephalitis.
The variables studied were age, sex, the reason for consultation, delay of consultation, weight and height, reason for hospitalization, paraclinical data (blood count, parasites count), the different clinical forms of malaria, and the length of hospitalization.
In operational definition:
- According to the WHO, adolescence is the period between childhood and adulthood, i.e. between 10 and 19 years of age. The United Nations Convention on the Rights of the Child defines childhood as the period from birth to 18 years (UNICEF 17). As an adolescent, we have retained, per the civil code of Gabon, a child in the age group of 10 to 17 years (until the day before the 18 th birthday). Children were defined as those between 1 month and 9 years (the day before the 10 th birthday).
- Severe malaria-related anaemia is defined as a haemoglobin level of < 5 g/dL in children under 12 years of age and < 7 g/dL in those over 12 years of age.
Hyperparasitemia is defined as a level of infected red blood cells > 20%
- Thrombocytopenia was defined as a platelet count < 150000 elements/mm 3
- The delay to consultation was "short" when it was less than the overall median duration.
Data were collected on Epi Info 7.2.2. A descriptive analysis was performed to determine the characteristics of the sample. Frequencies and prevalence were estimated with a 95% confidence interval. Quantitative data were averaged for biological endpoints, median and interquartile range for delay to consultation, age of children and length of hospital stay. Categorical data were expressed in frequency. To compare them, we used Chi-square independence tests. We used the Wilcoxon-Mann-Whitney test to compare the medians of the different groups and the ANOVA or Student's test to compare the means.
We performed univariate analyses (odds ratios - ORs) to assess the determinants of adolescent malaria in our context. The threshold for statistical significance was set at p < 0.05. The analysis was carried out using the online biostatistical software Pvalue ( https://www.pvalue.io/fr/ ), and we drew the graphs in MS Excel.
During the whole study, the entire process respected the ethic rules according the Helsinki Declaration revised in 2013.
During the period of hospitalization, we hospitalized 1734 patients, 689 for malaria, i.e. an incidence of 39.7% (95% CI [37.4%-42%]), including 128 adolescents. We rated 417 (24.3%; 95% CI [22.3%-26.3%]) cases of severe malaria, including 94 (22.5%; 95% CI [18.5%-26.6%]) adolescents, i.e. 73.4% (95% IC [65.8-81.1%]) of severe malaria among adolescents hospitalized for malaria during this period (Figure 1).
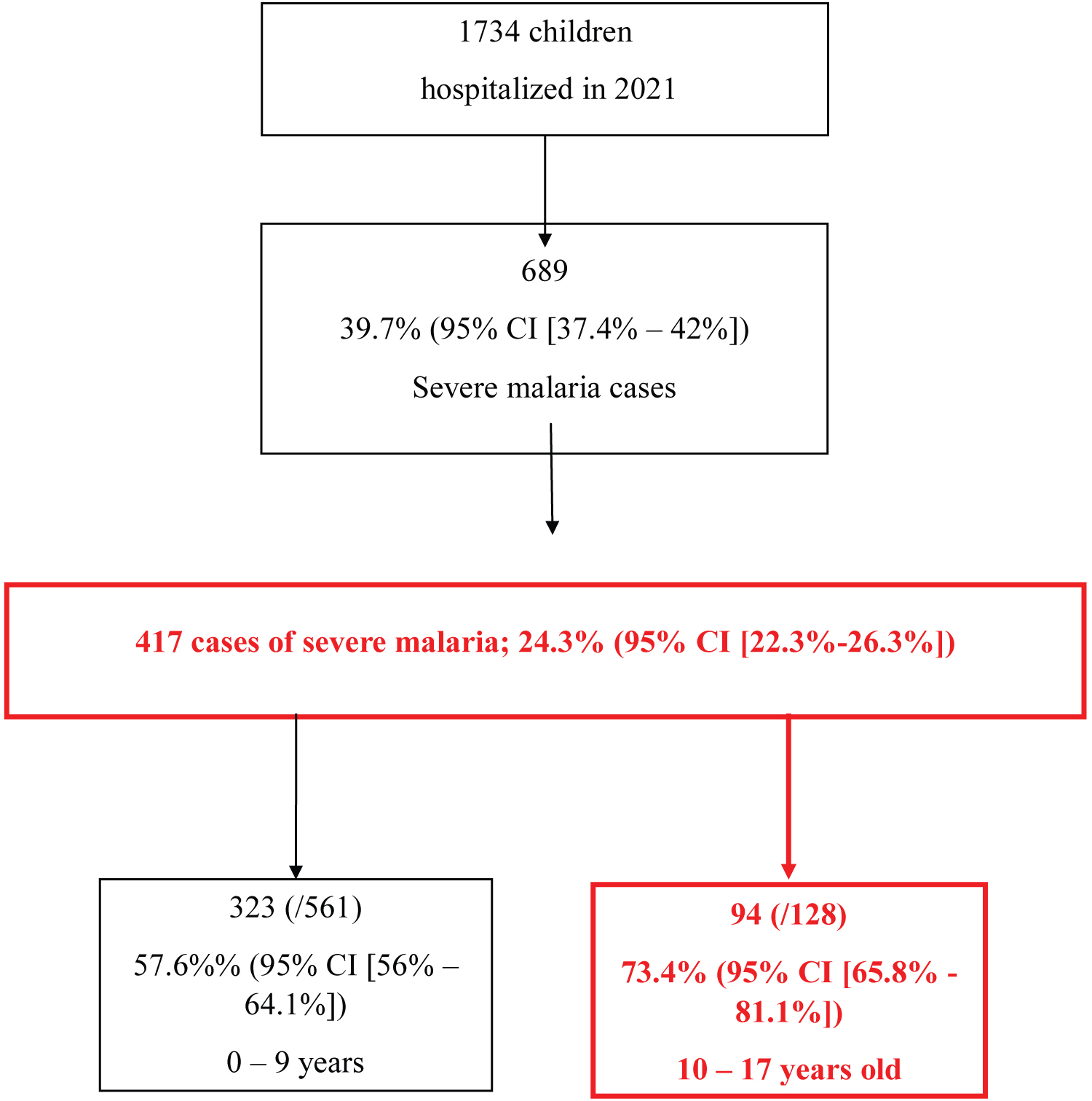 Figure 1: Flowchart of the subjects included in our study.
View Figure 1
Figure 1: Flowchart of the subjects included in our study.
View Figure 1
Of the 689 malaria cases, 311 were boys and 378 were girls. Of the 417 cases of severe malaria, 189 were boys, 228 were girls (p = 0.90).
The overall mean age was 6.1 ± 5.1 years, a minimum of 3 months and a maximum of 16 years. The median age of adolescents was 12 years, a minimum of 10 years and a maximum of 16 years. The median age of children was 3 years, a minimum of 3 months, and a maximum of 9 years.
The overall median delay to the consultation was 3 days. The median delay to consultation for adolescents was 2 days, and the median delay to consultation for children was 3 days p = 0.004 (Wilcoxon/Mann-Whitney test).
The main reasons for consultation were 98% fever among adolescents, followed by prostration in 72% (Figure 2).
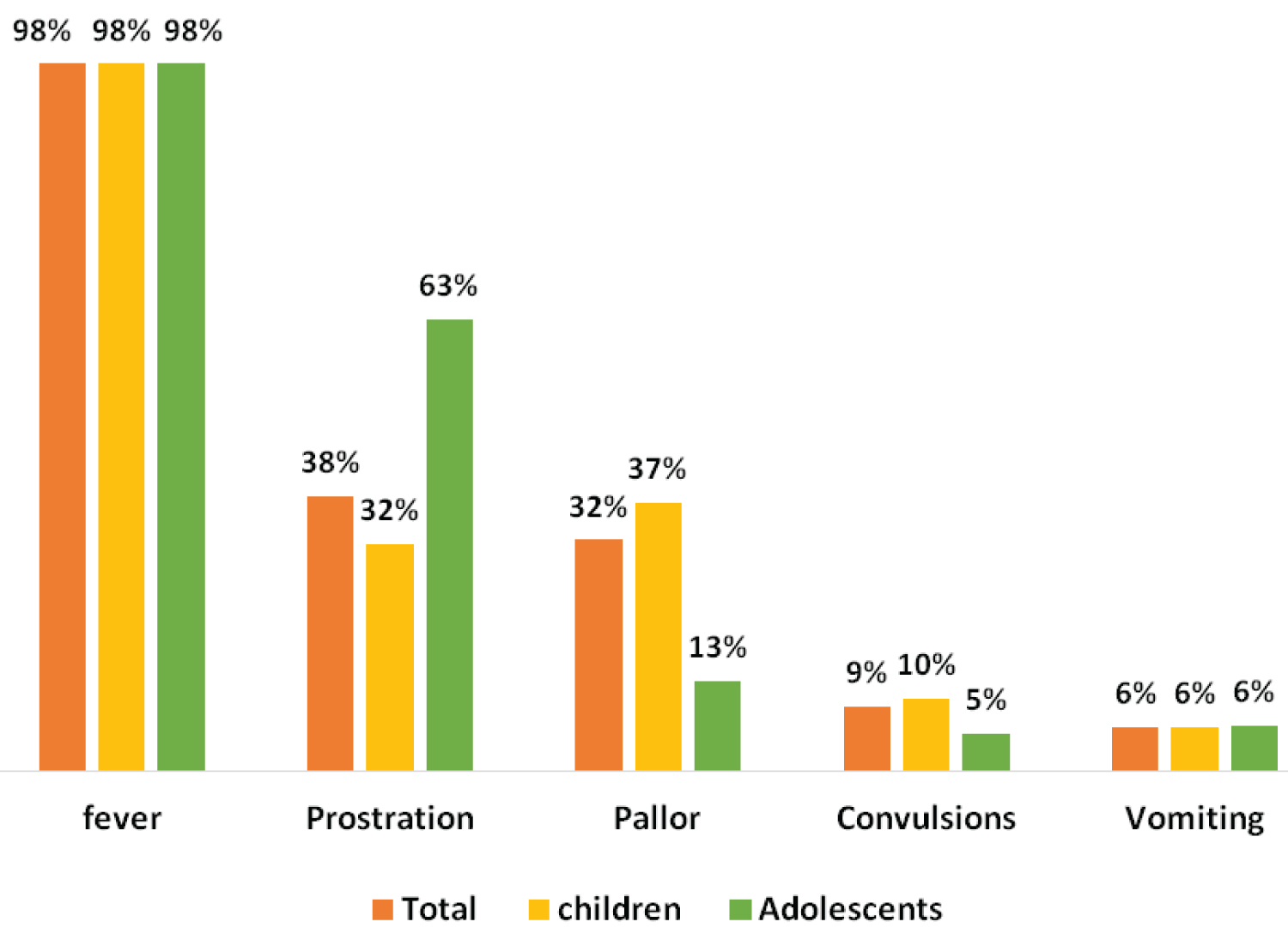 Figure 2: Distribution of the main signs and symptoms that led to the subjects' consultation.
View Figure 2
Figure 2: Distribution of the main signs and symptoms that led to the subjects' consultation.
View Figure 2
Severe anaemia was in 37.4% (n = 156/417; 95% CI [32.8%-42.1%]) of all subjects. The proportion of adolescents with severe anaemia was 17% (n = 16/94; 95% CI [9.4%-24.6%]), the proportion of younger children with severe anaemia was 43.3% (n = 140/323; 95% CI [30.5%-49.5%]), p < 0.001 .
The mean platelet count of all subjects was 137520 platelets/mm 3 (± 114165), and thrombocytopenia was 66.2% (n = 276/417; 95% CI [61.6%-70.7%]) of subjects. The proportion of adolescents with thrombocytopenia was 70.2% (n = 66/94; 95% CI [61%-79.5%]), the proportion of children with thrombocytopenia was 65% (n = 210/323; 95% CI [59.8%-70.2%]), p = 0.018 .
The mean parasitaemia of all subjects was 86000 parasites/mm 3 (± 132000), the minimum was 47 parasites/mm 3 , and the maximum was 735000 parasites/mm 3 . Hyperparasitemia occurred in 17% (n = 71/417; 95% CI [13.4%-20.6%]) of subjects. The proportion of adolescents with hyperparasitemia was 8.5% (n = 8/94; 95% CI [2.9%-14.2%]), the proportion of children with hyperparasitemia was 24% (n = 63/323; 95% CI [15.2%-23.8%]), p = 0.016 .
The main clinical severe form found in adolescents was the neurological form, with 72.3% (n = 68/94; 95% CI [63.3%-81.4%]). The proportions of the other primary severe forms are shown in Figure 3.
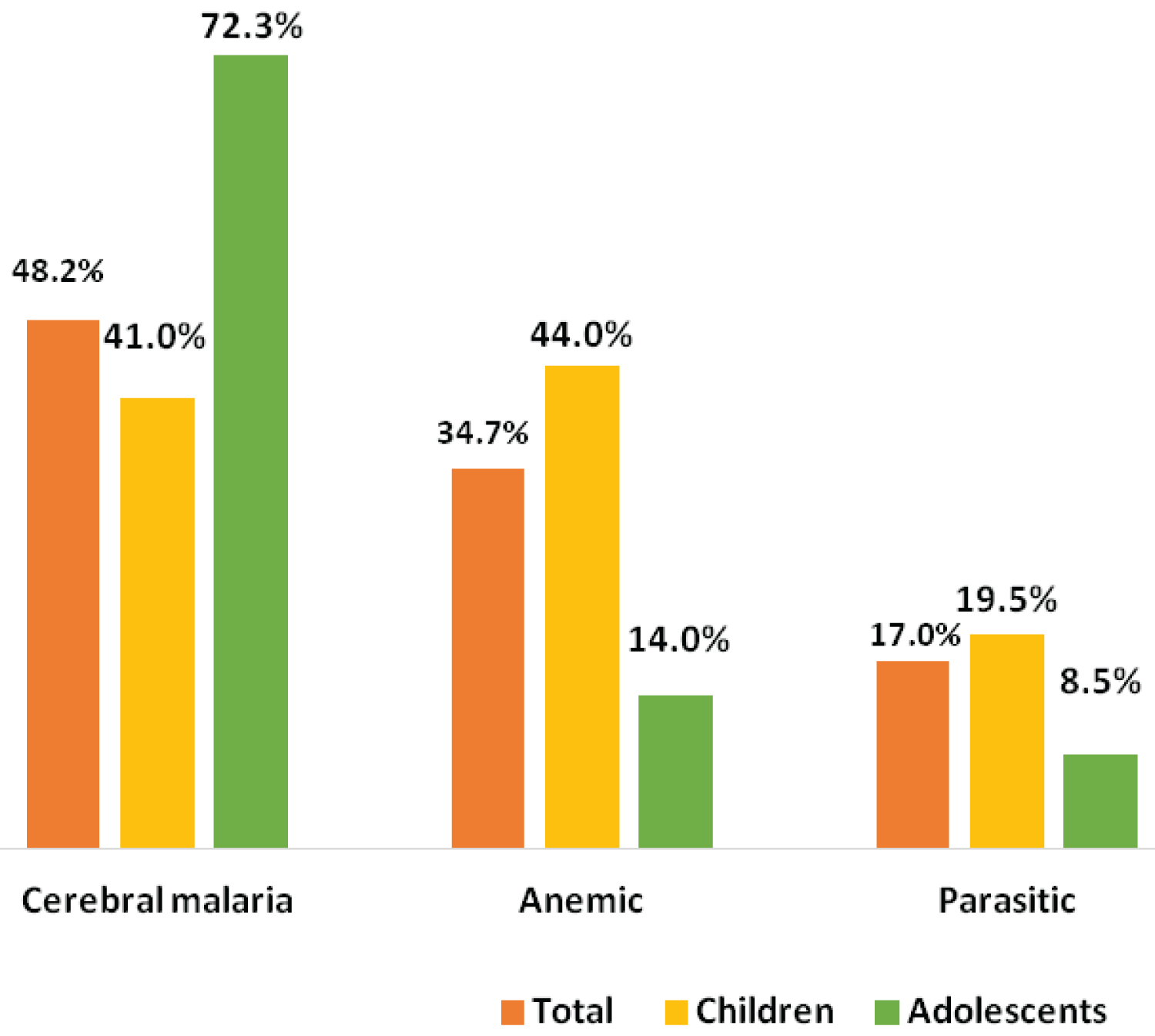 Figure 3: Distributions of severe forms of malaria in children.
View Figure 3
Figure 3: Distributions of severe forms of malaria in children.
View Figure 3
The median length of hospital stay for all children was 4 days, the median length of stay for adolescents was 3 days, and the median length of hospital stay was 4 days ( p = 0.0263 ).
We did not record any deaths during the study period.
Adolescent status was significantly associated with cerebral malaria and a short delay to consultation, as shown in Table 1.
Table 1: Factors associated with severe adolescent vs. infant malaria. View Table 1
The incidence of malaria in our ward was 39.7% in the year following the Covid-19 pandemic. This estimate does not reflect the population prevalence, as it should be noted that during this period, all health systems and programs were disrupted [6]. It is reported that during stable periods, in Central Africa, only 6 out of 10 febrile children are taken to the health centre, and less than 3 out of 10 are tested for malaria [6,8]. The post-Covid-19 period has been marked in our region by a decrease in the number of visitors to healthcare providers for fear of contamination and by mistrust of decision-makers [6]. Our prevalence is above the values that followed the implementation of malaria prevention measures in children under 5 years of age, with a low rate of 15% in 2008 in the same hospital [4]. An upward trend in malaria infections had already been noticed in 2011, with a rate of 24.1% [5]. More recent results show that malaria accounted for 36.3% of febrile illnesses hospitalized in another hospital in Libreville [2] and 40% in southeastern Gabon [9]. These results, which are close to our results and recorded after our survey, attest to the constancy of the current high incidence of malaria in febrile children in Libreville. In southeastern Gabon, an upward trend has been observed, with an increase in the frequency of malaria among children from 26.1% in 2019 to 58.8% in 2023, especially in urban areas [10,11]. These results reflect a lessening in our country's malaria prevention measures, a relaxation that began before the global health crisis. As an illustration, the latest UNICEF report on malaria mentions data on malaria-related mortality in Gabon but does not have information on the use of long-lasting insecticide-treated nets [8].
Depending on the age group, inpatient malaria is dominated by younger people. This distribution has not changed. Young children constitute the bulk of the contingent of patients treated for malaria in our context, with 79.4% in 2012 [11]. Adolescents had more severe forms than younger children. We noted that 74.3% of adolescents had severe malaria, while among the youngest; the proportion was lower, with 57.6% of cases. Overall, adolescents were more likely to develop severe forms of malaria than younger children. Considering that the delay to the consultation was shorter in adolescents than in younger children, the question of the higher rate of severe forms in adolescents arises more acutely. One explanation could be the lower platelets count in adolescents. Adolescents had proportionally more cases of thrombopenia, yet thrombocytopenia is described as predictive of severe malaria [11,12]. Another explanation could be in the behaviour of teenagers: They are often out of the house, therefore exposed to more bites, and would only declare their discomfort to their parents later. This phenomenon would be accentuated during the post-Covid 19 periods [6]. It can be inferred from this that at the time of diagnosis. We note that the pandemic and the period that directly followed were characterized by a disorganization of health systems, with a notable impact on the diagnosis and management of malaria in Africa [13].
The reasons for consultation were dominated by fever, followed by prostration. Fever is the main sign of malaria, and it is from its discovery that diagnostic tests are carried out to distinguish malaria from other etiologies [8,11]. Prostration was found in 38% of cases, including 63% of adolescents and 32% of younger children. Prostration is a sign of malaria severity, signing, along with convulsions and coma, the severe neurological form of malaria [1,14]. The survey by Imboumy-Limoukou, et al. conducted during the same period as ours in the southeastern region of Gabon, shows that neurological disorders are present in 92.27% of children hospitalized for severe malaria [15]. They were present in 43% of cases in the study by Ledongo Wombo, et al. and 41% in the study by Bouyou, et al. [9]. Neurological disorders, therefore, appear to be the most frequent clinical signs of severe malaria of all classes. The abundance of neurological troubles could find an explanation in the fact that neurological disorders are linked to both the pathophysiology of cerebral malaria on the one hand and anaemia on the other, both leading to cerebral hypoxia [16]. Cerebral forms seem to be generally increasing in our hospital, from 24% in 2005 to 23.7% in 2012 to 48.2% in our series [11,17]. Adolescents were nearly 4 times more likely to have severe cerebral forms than children. One potential explanation for this observation is the probable delay in consultation linked to adolescents' behaviour, which would, thus, cause the disease to evolve from a simple form to a more severe form [6]. The period of the pandemic has also led to a mistrust of the population about healthcare structures [6,13].
Overall, adolescents recovered more quickly than children. Their hospital length of stay was significantly shorter than that of children. The Gabonese national guidelines impose the treatment with injectable artesunate in the first line for severe malaria. All the children receive the same treatment [18]. This treatment is administered for 24 hours and prolonged in case of necessity until 72 hours (3 days). Therefore, we think a more extended stay in younger children may result in transfusion. Anaemia and severe anaemia were more frequent in younger children than in adolescents. The control of haemoglobin level after transfusion and the survey of transfusion tolerance prolonged the stay.
A lessening in the use of health structures and malaria control has marked the period of the Covid-19 pandemic. This reduction can be seen in our hospital by an increase in the incidence of malaria, but especially in severe forms. Adolescents in the immediate post-Covid-19 period, seen in our department, presented more neurological forms than other forms. Consequently, the sequelae linked to this form must be monitored to assess the real impact of covid-19 on children in our regions in the medium term.
All authors declare that they have no conflicts of interest in this publication.
The team thanks Dr Sylvie Mpira for her unique, precious and countless deployment on the field during this study.