Relationship between environmental pollution and lung cancer has not been fully clarified. There are particles with aerodynamic mass below 2.5 m, capable of directly injuring the lung due to their small size. The majority arise from the burning of fossil combustibles, and are called "residual oil fly ash" (ROFA). They have been demonstrated to damage cells in different ways; however, their relationship with lung cancer etiology has not been clarified. ROFA's influence on the development of lung cancer in mice, has been presently studied in male Balb-C mice exposed to daily nasal instillation of 10 μL of saline solutions, containing or not, 30 μg ROFA, for periods of 5, 9, 16, 26 weeks, or for 16 weeks to a daily dose of 120 μg ROFA. Quantitative analysis for the presence of hyperplasia and pulmonary nodules was performed in all animals. Morphometric analysis of the nodules from animals exposed to a daily dose of 30 μg ROFA were also made. Morphometric evaluation demonstrated that mice exposed daily for 26 weeks to ROFA, presented a higher Nuclear Fraction (p = 0.008) and a Stromal Fraction lower (p = 0.009) than those of the saline controls. In the animals exposed to 120 μg ROFA per day that presented significant inflammatory infiltration, a higher number of nodules (p = 0.045) and lung hyperplasia (p = 0.001) than those ones without inflammatory response. These alterations suggest that at low doses, ROFA presents subtle carcinogenic effects, evidenced by morphometric alterations, but that following administration of high doses, ROFA has carcinogenic effect associated with lung inflammatory response in mice.
Lung neoplasm, Air pollution, ROFA, Animal model
The present work has been reviewed and approved by the Committee for Control of Animal and Human Research Experimentation of the School of Medicine of São José do Rio Preto (FAMERP), of the State of São Paulo, Brazil.
Lung cancer is at present of high incidence in the world (12.9% of all new cases) arising as the most frequent cause of cancer death [1], with a prospective of becoming the fifth general cause of death in 2020 [2].
The relationship between lung cancer and environmental pollution is clear [3-5]. However, there remain many doubts about which components of atmosphere pollution possess a direct relationship with this neoplasm's development.
Environmental air contains an immense variety of known carcinogens present in the form of chemical compounds of various types. Their formation follows mainly the combustion of fossil fuels used to generate energy in industry and means of transportation.
Among these components, smaller particle materials with aerodynamic masses below 2.5 μm (MW2.5) which, due to their small size can directly reach the pulmonary alveoli present a higher capacity of injury.
This particulate material is not a sole entity. It comprises various classes of polluents, in their major part arising as residual oils from the combustion of fossil fluids. This originated the definition of "residual oil fly ash" or "volatile particles of residual oil" for them [6].
The products of this combustion are of organic and inorganic nature. Their analysis reveals the presence of complex chemicals that include sulfates silicates, as well as carbon, hydrogen and additive compounds. Metals like iron, vanadium and nickel are present at high concentration in the form of water-soluble salts.
Thus, ROFA becomes a very useful instrument for the study of biological effects of atmospheric pollution consequent to the presence of environmental particulate materials.
The mechanism by which ROFA induces lesions in cells and tissues is not fully understood. In normal animals it can originate inflammatory lesions accompanied by air pathway hyperreactivity and increased susceptibility to infection [7].
In humans, effects of ROFA include bronchitis, ocular irritation, sore throat, coughing, dyspnea, rhinitis, conjunctivitis as well as decreased pulmonary function [8-10]. The literature does not describe in clear-cut fashion their relation to pulmonary cancer.
To evaluate the effects of ROFA on the development of experimental lung cancer in mice.
Based on earlier work, we set up an experimental project using male, adult Balb-C mice aged between seven and thirteen weeks, kept in cages at 23 ℃ ± 2 and a relative air humidity of 60%. Animals were submitted to 12 h under light and 12 h in the dark; their diet was based on water plus a food ratio ad libitum.
On the first day of the experiment, all animals were weighed, and intraperitonialy injected with 3.0 mL/kg body weight, of a solution of 10 g of urethane in 100 mL of 0.9% of NaCl, divided into two equal doses given 48 h apart.
Urethane, the ethyl ester of carbamic acid, is a carcinogen, widely utilized in studies of experimental chemically-induced carcinogenesis, capable of producing for example, lung adenocarcinoma in mice [11,12]. It is adequate for the development of a tumor within a short time period and shows low toxicity, associated to a reduced animal mortality.
The exposure to the test substances was made via nasal instillation of 10 μL of a 0.9% saline solution containing or not ROFA, collected and processed by the Environmental Pollution Laboratory of the University of S. Paulo (USP). ROFA is PM collected was collected from the solid waste incinerator of the University Hospital of the University of São Paulo, which is powered by combustible oil. The element composition of ROFA was determined by neutron activation analysis and it is homogeneous and rich in water-soluble transition metals [13]. The following experimental groups were set up:
Study 1: Four groups receiving a daily dose of 30 μg of ROFA for 5, 9, 16 and 26 weeks as well as corresponding controls treated only with saline.
Study 2: Two groups dosed with 120 μg of ROFA, subdivided in two daily doses for 16 weeks, as well as their saline controls.
We decided empirically to test these two doses based on previous protocols as in Ghio, Arantes-Costa, Bizelli [6,14,15] and others pilot studies, not published.
Animals belonging to the first group were sacrificed 16 weeks following the beginning of the treatments. This period was chosen based on earlier work of our research group [11,12,16]. Animals pertaining to the group exposed to ROFA for 26 weeks were sacrificed at the end of this period.
Following these treatments, animals were weighed, ether-anesthetized and sacrificed by bleeding, following section of the abdominal aorta. Their lungs were removed, inflated and fixed by intratracheal instillation of a buffered 10% formalin solution.
Microscopic quantitative analysis of hyperplasia and pulmonary nodules was made following slide dyeing with hematoxylin-eosin.
The following criteria for the distinction between nodule and hyperplasia were adopted: A nodule was so considered if the group of cells showing alteration suggestive of neoplasm was higher or equal to the area of five alveolar spaces; if it was lower that, hyperplasia was considered [12].
Besides the lungs, the following organs were also histologically evaluated: Spleen, liver, kidneys, heart and thymus, by searching for morphological alterations like metastases or inflammation. Following these analyses, animals of the group exposed to the daily dose of 30 μg ROFA and their controls were morphometrically analyzed. Five animals of each group were studied using the point counting technique [17], with the aid of a coherent reticle of points and straight lines.
Five randomly digitized slide fields obtained from tumor regions, were studied under 400-fold magnification, totaling 500 points per case. Every point of intersection of the reticle was evaluated, searching to classify it for its tumor projection into nucleus, cytoplasm or stroma.
The analyses covered the Stromal Fraction (Str. F.), the Nuclear Fraction (Nuc. F.) and the Cytoplasm Fraction (Cyt. F.); the relation between nucleus/cytoplasm (N/C), as defined below, was also studied:
1) Str.F. = Str. P./Tumor. P.
Where Str.P. and Tumor.P are respectively, number of points reaching the stroma, or the tumor. The same method was utilized to calculate the other parameters:
2) Nuc. F. = Nuc. P./Tumor.P.
3) Cyt. F. = Cyt. P./Tumor. P.
4) Nuc/Cyt = Nuc. P./Cyt. P.
An error coefficient below 5% was maintained for all morphometric parameters. All results were statistically evaluated utilizing non-parametric methods of the Mood-median [18].
The final number of animals in each group is presented on Table 1.
Table 1: Final numbers of animals in each study group. View Table 1
The histological study of the lungs revealed the presence of neoplastic nodules and hyperplasia, as well as the deposition of anthracotic pigment in the lungs of mice exposed to ROFA (Figure 1). Chemical analyses of the components of the ROFA utilized in this study are described on Table 2, Table 3 and Table 4.
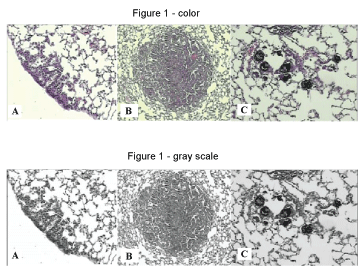 Figure 1: Microscopic appearance of mice lung. A: Lung hyperplasia (Hematoxilin-Eosin - 100X) (USG 16); B: Lung nodule (Hematoxilin-Eosin - 40X) (USG 16); C: Deposition of anthracótic pigment in pulmonary alveoli (Hematoxilin-Eosin - 100X) (URG26).
Figure 1: Microscopic appearance of mice lung. A: Lung hyperplasia (Hematoxilin-Eosin - 100X) (USG 16); B: Lung nodule (Hematoxilin-Eosin - 40X) (USG 16); C: Deposition of anthracótic pigment in pulmonary alveoli (Hematoxilin-Eosin - 100X) (URG26).
URG 26: (Urethane + ROFA 30 μg/day Group) for 26 weeks; USG 16: (Urethane + Saline Group) for 16 weeks. View Figure 1
Table 2: Polycyclic Aromatic Hydrocarbon (PAH) concentrations. View Table 2
Table 3: Concentration of organochlorides in ROFA determined by chromatography. View Table 3
Table 4: Concentrations of chemical elements in ROFA. View Table 4
No statistically significant differences were demonstrated between the number of hyperplasia and of pulmonary nodules of these groups (Figure 2 and Figure 3). Morphometric analysis of groups exposed to ROFA during 5, 9 and 16 weeks respectively, did not reveal statistical differences between the parameters evaluated.
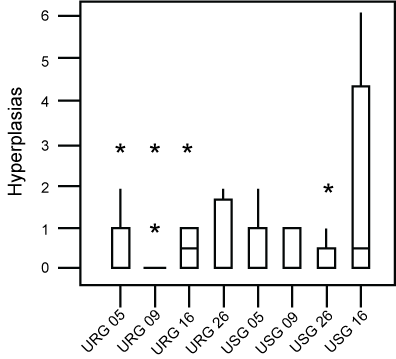 Figure 2: Box-plot of the distribution of hyperplasia found in each group. No difference was found between groups. Asteriks are outliers.
Figure 2: Box-plot of the distribution of hyperplasia found in each group. No difference was found between groups. Asteriks are outliers.
URG: (Urethane + ROFA 30 μg/day Group) for 5, 9, 16 or 26 weeks; USG: (Urethane + Saline Group) for 5, 9, 16 or 26 weeks. View Figure 2
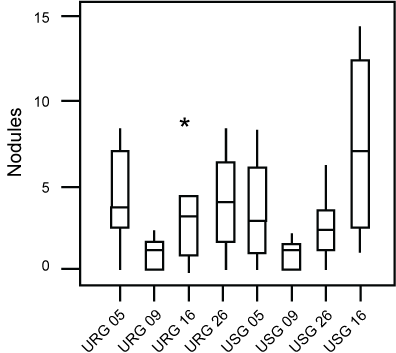 Figure 3: Box-plot of the distribution of nodules found in each group. No difference was found between groups.Asteriks are outliers.
Figure 3: Box-plot of the distribution of nodules found in each group. No difference was found between groups.Asteriks are outliers.
URG: (Urethane + ROFA 30 μg/day Group) for 5, 9, 16 or 26 weeks; USG: (Urethane + Saline Group) for 5, 9, 16 or 26 weeks.
View Figure 3
However, the evaluation of the animals exposed for a longer period (26 weeks) to ROFA, showed them to exhibit a statistically higher Nuclear (p = 0.008), as well as a lower Stromal Fraction (p = 0.009), following comparison to the group exposed to saline alone (Figure 4 and Figure 5).
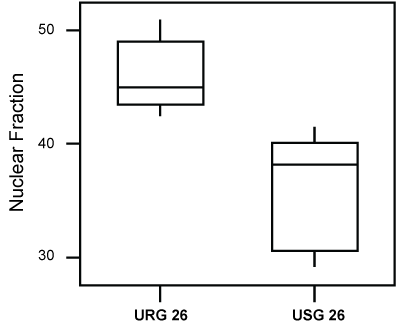 Figure 4: Box-plot of the Nuclear Fraction values of groups USG26 and URG26. The values of the nuclear fraction were higher (P = 0.008), in the group exposed to ROFA.
Figure 4: Box-plot of the Nuclear Fraction values of groups USG26 and URG26. The values of the nuclear fraction were higher (P = 0.008), in the group exposed to ROFA.
URG: (Urethane + ROFA 30 μg/day, group) for 26 weeks; USG: (Urethane + Saline Group) for 26 weeks. View Figure 4
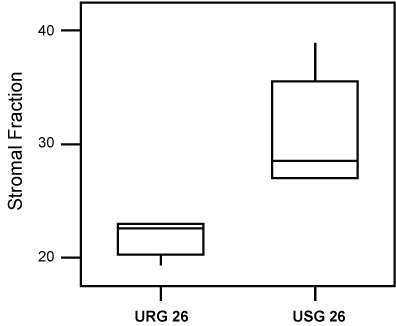 Figure 5: Box-plot of the values of the Stromal Fraction of groups USG26 and URG26. Lesser values (P = 0.009) of the Stromal Fraction, were observed in the group exposed to ROFA.
URG: (Urethane + ROFA 30 μg/dia, Group) for 26 weeks; USG: (Urethane + Saline Group) for 26 weeks. View Figure 5
Figure 5: Box-plot of the values of the Stromal Fraction of groups USG26 and URG26. Lesser values (P = 0.009) of the Stromal Fraction, were observed in the group exposed to ROFA.
URG: (Urethane + ROFA 30 μg/dia, Group) for 26 weeks; USG: (Urethane + Saline Group) for 26 weeks. View Figure 5
The evaluation of the number of nodules and of hyperplasia did not reveal statistically significant differences between those groups (P = 0.847 and P = 0.426 respectively); however, we noted that five animals of the ROFA group, presented a significant lymphocyte inflammatory reaction, predominant at the broncho-alveolar peri-interstice A higher deposition of anthracotic pigment in the lungs of these animals was also observed.
When we performed statistical comparisons between the number of nodules and hyperplasia in animals showing an inflammatory reaction and the remaining animals, we found a higher incidence of nodules (P = 0.046) and hyperplasia (P = 0.001) in the former group (Figure 6 and Figure 7).
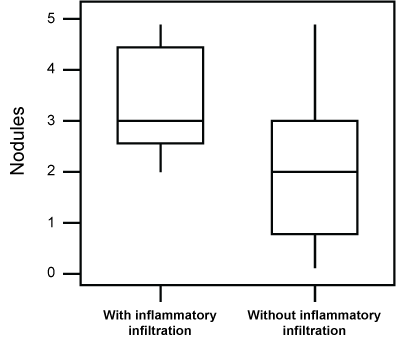 Figure 6: Box-plot of the incidence of pulmonary nodules in animals exposed to a daily dose of 120 μg ROFA for 16 weeks, presenting an inflammatory infiltrate, in comparison to the remaining ones. A higher value (P = 0.046) of nodules was seen in animals showing the infiltrate. View Figure 6
Figure 6: Box-plot of the incidence of pulmonary nodules in animals exposed to a daily dose of 120 μg ROFA for 16 weeks, presenting an inflammatory infiltrate, in comparison to the remaining ones. A higher value (P = 0.046) of nodules was seen in animals showing the infiltrate. View Figure 6
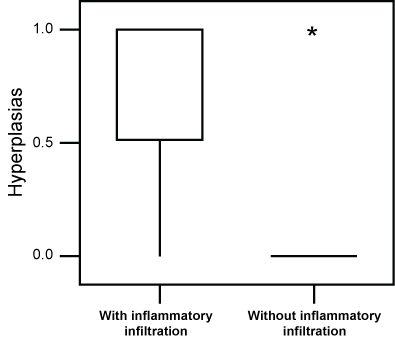 Figure 7: Box-plot of the incidence lung hyperplasia in animals exposed to a daily dose of 120 µg ROFA for 16 weeks presenting an inflammatory infiltrate, in comparison to the remaining ones. A higher value (P = 0.001) of hyperplasia was seen in animals showing the infiltrate. Asteriks are outliers. View Figure 7
Figure 7: Box-plot of the incidence lung hyperplasia in animals exposed to a daily dose of 120 µg ROFA for 16 weeks presenting an inflammatory infiltrate, in comparison to the remaining ones. A higher value (P = 0.001) of hyperplasia was seen in animals showing the infiltrate. Asteriks are outliers. View Figure 7
Our study demonstrated the existence of some alterations suggesting that the exposure to ROFA influences the development of experimental lung cancer in mice. In low doses, alterations of the morphometry were noted, although in higher doses, alterations in the number of pulmonary lesions, associated with a lymphocytic inflammatory process could be observed.
Morphometric analyses were performed with the purpose of detecting subtler alterations evoked by ROFA in the development of the pulmonary neoplasm. Variations in the nuclear, cytoplasm and stroma of a tumor, can represent variation in its degree of malignancy, its differentiation and its speed of progression [19,20].
Previous studies had evidenced that the stromal fraction is an important morphometric marker for the relationship of the effects of pollution on lung tumors [11]. Its decrease could be a consequence of the direct action of polluents on the extracellular matrix, an alteration also observed in the present work.
The role of the extracellular matrix in neoplasms has recently raised great interest. One of its proposed mechanisms would be a decrease of type IV collagen and an increase of type I collagenase activity; another would be alterations in the expression of integrins, possibly associated to metastatic growth [21].
Size and nuclear characteristics are of great importance for neoplasm evaluation since they are indirectly related to morphologically undetectable genetic and molecular alterations [22]. The increased nuclear fraction following prolonged exposure to ROFA strengthens the hypothesis that pollution accelerates the evolution of neoplasms. It therefore appears that the morphometric alterations found, may be an indirect indication of tumor malignancy [11].
In our study we noted that mice exposed to doses of 120 μg ROFA/day, showing lymphocyte inflammation of the lung upon microscope analysis, presented a higher deposition of anthracotic pigment and development of a larger number of nodules in comparison with the lung of mice not showing such inflammatory response.
Some studies in the literature demonstrate the presence of inflammation of air pathways following exposure to ROFA particulate material, capable to stimulate an increase of pro-inflammatory substances. Among these, cytokines, proteokinases, increased mRNA transcription of macrophage inflammatory proteins, interleukins-6 and the tumor- necrosis factor stand out [23].
Also the persistence of the controversy regarding the validity of intratracheal instillation of particulate matter as a surrogate of inhalation exposure in rodents motivated some other studies. Costa and colleagues [24] demonstrated that intratracheal instillation and inhalation exposure proportionate a similar distribution of the ROFA markers (vanadium and nickel), with exception of the inferior lobe dose (where the concentration of ROFA markers were approximately 25% bigger in intratracheal instillation). Alveolitis and bronchial inflammation or epithelial hypertrophy were prominent 24 hours following instillation, but it was not apparent after inhalation. Alveolar hemorrhage, congestion and airway exudates were pronounced at 48 hours post inhalation, but not important in the instillation-group.
Nurkiewicz, et al. [25] purposed to characterize the effect of pulmonary particulate matter exposure on systemic microvascular responses beyond identify the local inflammatory events that may contribute for these events. After intratracheal instillation in rabbits using residual oil fly ash (ROFA) or TiO2, it was observed that the damage of systemic endothelium-dependent arteriolar dilation coincides with the polimorphonuclear leukocyte adhesion, hemoprotein deposition and local oxidative stress. These observations are coherent with the events with contribute to the loss of control of the peripheral resistance and de cardiac dysfunction associated with the particulate matter exposition.
Prominence has also been given to the expression of pro-inflammatory genes, like 8-oxo-dG [23] and to the presence of oxidative stress as elements possibly directly connected to carcinogenesis [5,23,26-28]. According to Grivennikov and cols [29], many environmental causes of cancer and risk factors are associated with some form of chronic inflammation and 30% can be attributed to tobacco smoking and inhaled pollutants .
The fact that we did not observe the process of carcinogenesis in all other animals treated with ROFA can have several explanations. One is the possibility of the existence of genetic alterations or other forms of resistance, leading to a decreased inflammatory response to ROFA or even to urethane. Genetic alterations causing a smaller inflammatory response in mice have been described [30,31].
Experimental models may show maximal (AIRmax) or minimal (AIRmin) capacity to present an acute inflammatory response, like strains of the AIR Selection ("Acute Inflammatory Reaction") [32], or of high or low levels of antibodies against complex antigens of strains H ("High") and L ("Low") of the Selections I, II, III, IV [33].
An interesting road to follow in order to verify the relation of the inflammatory process due to inhalation of polluents like ROFA, in animals more or less susceptible to inflammation, would be its association with the possible promoter effect of urethane-induced carcinogenesis. Two non-isogenic strains of mice phenotypically selected for maximal (AIRmax) or minimal (AIRmin) capacity, showing acute inflammatory response (AIR), present differential susceptibility to chemical lung and skin carcinogenesis [34-37]. The AIRmax strain is resistant, while the AIRmin strain is susceptible, and several indications that a genetic regulation shared between phenotypes of acute inflammatory reactivity and predisposition towards chemical carcinogenesis exists.
At any rate, despite the fact that few animals had presented inflammation and consequent development of tumor nodules, the relationship between inflammation and cancer has been much investigated [34-37], its results aiding to the understanding of this as yet little studied, but very important theme. Increases in the dose of ROFA here employed, to verify the possibility of obtaining a more intense inflammatory stimulation, would be an interesting lead towards further research.
The alterations found in this study, suggest.
We thank the Experimental Air Pollution Laboratory of the University of São Paulo, São Paulo, Brazil, in the figure of Professor Dr. Paulo Hilário Saldiva, for the availability of ROFA collected from the incinerator of the Hospital of the University of São Paulo, and the financial support of the "Conselho Nacional de Desenvolvimento Científico e Tecnológico" (CNPq) and of the "Fundação de Amparo à Pesquisa do Estado de São Paulo" (FAPESP).