Liver metastasis can be defined as the seeding of a malignant tumour from other organs affected by cancer. About half of the patients with colorectal cancer develop liver metastases during the progressive course of the disease. Surgery is the only curative treatment for patients with metastatic liver disease, and the five-year survival rate after resection of all detectable liver metastases can be as high as 40%. Unfortunately, only 25% of patients with liver metastases are candidates for liver resection. Regional treatments such as radiofrequency ablation and cryotherapy may be offered to patients with isolated, unresectable metastases but no evidence of extrahepatic disease. Chemotherapy and chemoembolization are often used in patients with liver metastases, but patients with extrahepatic disease are not suitable candidates for regional ablation. For patients with metastatic colorectal cancer beyond the liver, systemic chemotherapy is a better treatment option. Immunotherapy, when used with chemotherapeutic agents, has an additive therapeutic effect. More than half of the patients die of metastatic liver disease. Surgical resection of distant metastases can lead to prolonged survival and cure in a selected group of patients. This article reviews the current concepts along with recent advances and future perspectives in the management of patients with liver metastasis.
Liver metastasis, Future liver remnant, Surgical resection, Chemotherapy, Radiofrequency ablation
The liver is a frequent target of hematogenous disseminated malignancies of the gastrointestinal tract, including secondaries from other affected organs, making the development of liver metastases more unique from an oncological point of view [1]. Several aspects of the anatomy and physiology of the liver make it particularly amenable to oncological therapy [2]. The nature of the portal venous drainage makes the liver a frequent target of hematogenous disseminated gastrointestinal malignancies, often postulated as a mechanical or hemodynamic hypothesis [3].
Liver metastases have a very different and unique growth pattern, and the challenges faced in the treatment are different from those of conventional liver tumours like hepatocellular carcinoma [4]. There is also squamous cell carcinoma, neuroendocrine carcinoma, and other much fewer common subtypes such as lymphoma, sarcoma, and melanoma. Overall, the vast majority of literature focuses on the treatment of colorectal adenocarcinoma, which is reported to be the third most common primary malignancy worldwide. Most importantly, 70-80% of the metastatic lesions will remain seated in the liver [5].
To determine the origin and incidence of liver metastasis in the Netherlands, more than 23,000 patients were examined between January 2001 and December 2010 for the association of tumour type, primary tumour origin, and patient characteristics with histopathology-confirmed liver metastasis. In this study, the most common metastases were shown to come from the colorectal region, followed by the stomach and breast.
Metastatic liver disease is more common in women younger than 50 than breast cancer. By age 70, 92% of metastatic liver lesions are from the GI tract. Among these carcinomatous lesions, 75% are adenocarcinomas. In general, histologically confirmed liver metastases are more common in men than women, and most are in their 50s. It is also reported that 20-25% of colorectal cancer patients will develop liver metastases, and 15-25% of these cases show synchronous metastases [5].
Understanding the surgical anatomy of the liver has facilitated safe liver surgery. Couinaud, a French surgeon, divided the liver into two hemi-livers, four sections, and eight segments [6]. Each of these segments can be considered a functional unit, with branches from the hepatic artery, portal vein, and bile duct and drainage from the branches of the hepatic vein [7]. It is the concept of the segment as a distinct functional unit that allows for "anatomical" liver resection [8]. The general anatomy of the liver is divided into right and left units, which run along the line between the gallbladder fossa and the middle hepatic vein, called the principle scissure (Cantlie's line). Determination can be made from individual segments or from the left or right hemi-liver [9]. Traditionally, the surgical strategy involved resection of the liver or liver segment containing the disease to achieve the largest negative margin [10]. Although anatomic resection remains the treatment of choice for HCC patients, a nonanatomic parenchymal sparing approach involving multiple metastasectomies is now considered the standard of care for those with colorectal liver metastases [11]. This approach does not affect long-term oncological results and spares the remaining liver, thus allowing more resections in case of disease recurrence [12].
The veins from the alimentary tract, pancreas, and spleen form the portal venous circulation. Back blood flow from this area is directed to the portal vein. The dual blood supply of the liver makes anatomical features even more unique. Hepatocytes receive most of their blood supply from the portal venous circulation, while cholangiocytes, which form the biliary system, receive most of their blood supply from the hepatic arterial circulation. More importantly, it is considered that primary and metastatic tumours in the liver usually receive blood supply from the hepatic arterial circulation.
The important physiological contrast of blood circulation between normal hepatocytes and abnormal cancer cells provides an opportunity for therapeutic interventions because most liver-directed therapies rely on embolic particles, chemotherapy, or radiation to the hepatic arterial circulation to target neoplastic cells and save hepatocytes. The high metabolic properties of hepatocytes also facilitate liver-directed therapy. Chemotherapeutic agents that first undergo hepatic transplantation can be delivered directly into the hepatic arterial circulation in exceptionally high concentrations with systemic effects and minimal hepatic cell metabolism toxicity [13].
Another unique physiological feature of the liver is its ability to hypertrophy in response to various insults and show complete regeneration within weeks. As long as the remaining hepatic veins and hepatic ducts are preserved, patients can tolerate up to 80% liver resection with normal liver function [14]. Similarly, hepatocellular ischemia resulting from a partial loss of portal vein flow results in a compensatory hypertrophic response elsewhere in the liver. In addition, in some patients who are amenable to aggressive surgical resection, inadvertent portal vein embolism of the affected segment may cause hypertrophy of the future liver remnant [15]. Liver-directed therapy utilises both of these physiological processes.
At least three different histological growth patterns have been observed in metastatic colonisation and growth in the liver, which include replacement, desmoplastic, and regrowth patterns. Two additional but rare histologic growth patterns are sinusoidal and portal. Each of these growth patterns differs from the corresponding characteristics at the tumour/normal liver interface, as discussed here. In addition, although this growth pattern was first observed using human patient samples, it was later demonstrated in a mouse model of liver metastasis. The variable histological growth pattern is characterised by cellular replacement of hepatocytes and swelling, the absence of desmoplastic stroma, and a nominal rate of proliferation of endothelial cells.
Liver metastases exhibit a variable growth pattern, tending to favour sinusoidal hepatic vasculature without relying on controlled angiogenesis, which contributes to resistance to anti-angiogenic therapy. This observation is significantly associated with a more accurate patient prognosis. Breast liver metastases with a breast tumour phenotype are more likely to have central hypoxia than metastases with an irreversible growth phenotype [16].
The desmoplastic histological progression pattern, on the other hand, is characterised by extensive stromal remodelling at the tumour-hepatocyte junction, along with significantly increased angiogenesis. This remodelling separates the normal liver parenchyma from the invading tumour cells by a layer of fibrotic stromal tissue. During this stage of histological development, there is no metastatic resemblance to normal liver architecture, and metastatic growth does not directly interact with normal hepatocytes.
In colon cancer liver metastases, the desmoplastic growth pattern associated with type I and IV collagen is associated with an increased proteolytic system, uPA-uPAR-PAI-1, which facilitates the interaction between the growing metastasis and the new tissue environment. Patients with desmoplastic colorectal liver metastases have a more favourable outcome compared with other histological types.
A benign histological pattern of progression, on the other hand, is characterised by the flattening and narrowing of hepatocytes with metastasis but no fibrosis. Similar to the desmoplastic histological growth pattern, the metastatic growth observed here does not resemble the cellular architecture of the liver. Histologically, proliferative induction was greater than desmoplastic or replacement patterns when examining endothelial and tumour cell sections, and this increase in proliferative potential correlated positively with overall metastasis size. The proliferative group is the most angiogenic of the histological growth patterns, and this angiogenesis appears to be partly caused by hypoxia [17].
Two additional histological growth patterns, sinusoidal and portal, are rare in BCLM. In sinusoidal growth, metastases present as emboli in the sinusoidal blood vessels of the liver and/or in the peri-sinusoid. Thus, sinusoidal blood vessels are used for metastatic vasculature and are found in more rapidly growing diseases. During the portal histological growth pattern, BCLM grows exclusively in the portal tract, hepatic capsule, and septa. Although these histological growth patterns are quite different from each other, it is important to note that they show considerable plasticity. For example, systemic antiangiogenic treatment of colorectal liver cancer metastases (CRLM) resulted in a phenotype switch from angiogenic desmoplastic growth to nonangiogenic transformation. This type of transition has been observed clinically in lung and brain metastases [18].
Furthermore, although patients with HER2+ disease are clearly enriched for BCLM, the subtypes of metastatic histologic progression are unclear. Frentzas, et al. The histological growth patterns shown in patient tumours were not statistically different between breast tissue subtypes, often reflecting phenotypes. Indeed, only one (Luminal B, HER2) of the seventeen samples showing a desmoplastic phenotype was excluded from this population [19].
Patients may present with the chief complaints of dull aching and continuous pain in the right upper abdomen, abdominal distension, loss of appetite, weakness, asthenia, malaise, jaundice of a shorter duration, mild, usually non-progressive and is not associated with pruritis (hepatocellular variety), encephalopathy, persistent vomiting with or without blood, severe backache without jaundice/Spine tenderness, jaundice first and associated with itching, metabolic disturbances, mass per rectum, altered bladder & bowel habits with bleeding per rectum, difficulty in breathing, history of similar illness or cancer related deaths in family, previous surgery such as mastectomy or amputation of toes, leg or wide excision of mole [20].
The patient may have classical findings of hepatic disease (caput medusa, hepatosplenomegaly, and ascites), a digital rectal examination (DRE), a protoscopy with a subsequent colonoscopy to check for masses and blood in the stool, as hepatic disease may arise elsewhere. As hepatic disease may arise elsewhere, a thorough physical examination, including inspection for any cutaneous malignant lesions, palpation for any lump in the breast, for lymphadenopathy, and thorough auscultation for adequate breath sounds, is very important in the decision-making process during treatment.
Sources for the secondaries include colorectal cancer, neuroendocrine tumours arising from the GI tract and pancreas, carcinomas of the breast, malignant melanoma, intrabdominal and retroperitoneal sarcomas, carcinoma of the gallbladder, small cell carcinoma of the lung, malignant lesions of the bones, carcinoma of the urinary bladder, carcinoma of the testis and prostate, carcinoma of the ovary, endometrium, and throid. Causes of bulky secondary cancers include malignant melanoma, carcinoid tumours, mucinous (colloid) carcinomas of the breast, lungs, pancreas, colon, or rectum, and in females, endometrial or ovarian cancer [21].
Like other adenocarcinomas of the alimentary tract, colorectal adenocarcinoma has a proclivity for hepatic metastasis. The liver is the most common site for metastasis in such cases. Colo-rectal cancer (CRC) represents a major worldwide health care burden as the 2 nd most common cancer diagnosed in women and the 3 rd most common in men. It is the major cause of morbidity and the second most common cause of mortality. Hence, in all such cases, liver-directed therapies are employed to achieve the desired treatment goals [22].
Like colorectal adenocarcinomas, neuroendocrine carcinoma arising from the gastrointestinal tract and pancreas commonly metastasizes to the liver via the portal venous circulation. The well-differentiated neuroendocrine carcinoma often progresses slowly, with median survival with even widely metastatic disease measured in years. Hormones released by neuroendocrine neoplasms in the liver escape hepatic metabolism; as a result, patients with hepatic metastases can suffer from syndromes of hormonal overproduction that can become refractory to pharmacologic suppression. An important property of neuroendocrine metastasis is that dissemination from such primary tumours is often diffuse in nature. Indeed, it is not uncommon to find innumerable liver lesions in patients with metastatic neuroendocrine carcinoma. Not surprisingly, the likelihood of performing a complete resection and the durability of hepatic recurrence-free survival are much lower for hepatic neuroendocrine metastases. This important difference informs decision-making when considering the various treatment options available for this disease. The carcinoid is the most common neuroendocrine tumour causing liver metastasis, especially when of midgut origin. Even though the appendix is the most common site for midgut carcinoids, they rarely metastasize to the liver. Similarly, hindgut carcinoid tumours are usually of rectal origin and, if less than 2 cm in diameter, rarely metastasize to the liver [23].
Nearly all fatalities arising from breast tumours are attributable to distant metastases. Breast cancer liver metastases have the worst prognosis, with an overall survival rate of 24-36 months. The tumour intrinsic subtype directs preferential metastasis to specific organs, with HER2-enriched tumours demonstrating the highest rates of metastasis to the liver, though all subtypes can grow in the liver [24].
Like colorectal adenocarcinomas and neuroendocrine carcinomas, ocular melanoma has a biological tendency to metastasize to the liver. The liver is the predominant site of metastatic disease in 70% to 90% of all the cases with diffuse or miliary patterns [25].
Unlike the primary tumours discussed earlier, intra-abdominal and retroperitoneal sarcomas occasionally metastasize to the liver. There are relatively few systemic chemotherapy options for patients with metastatic sarcoma, and liver-directed therapy is generally undertaken in selected cases that exhibit relatively favourable disease biology [26]. One important exception is gastrointestinal stromal tumours, which are likely to respond to the tyrosine kinase inhibitors imatinib and sunitinib. Not surprisingly, hepatic metastasectomy can be undertaken more aggressively in patients with metastatic gastrointestinal stromal tumours when used in conjunction with tyrosine kinase inhibition [27].
Liver imaging plays a very critical role in the clinical management of liver metastases. At the very least, the evaluation of patients with liver metastases requires high-resolution contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) [28].
Ultrasonography and positron emission tomography (PET) are used selectively, and somatostatin receptor scintigraphy is sometimes used in the evaluation of neuroendocrine carcinoma. Because of the relatively low sensitivity (64%) of CRLM, USG has a limited role in preoperative evaluation.
CEUS (Contrast Enhanced Ultrasonography) has been used more widely in recent years to characterise liver lesions based on a dynamic assessment of tumour vasculature. It has a sensitivity of 80-90% compared to CT and is more sensitive than grey-scale ultrasound for detecting CRLM smaller than 10 mm. However, CEUS does not provide the necessary information for surgical planning compared to CT or MRI.
IOUS (Operative Ultrasound) has played a role in identifying and mapping major hepatic artery lesions during surgery. IOUS has been shown to detect new lesions in 16% of patients and to change clinical management in 9% [29].
CEIOUS (Contrast Enhanced Intra-Operative Ultrasound) has higher sensitivity and specificity than conventional IOUS, especially for detecting "growing lesions" in the setting of neoadjuvant chemotherapy [30].
CECT (Gold Standard) uses three-phase imaging (consisting of a non-contrast phase, an arterial phase, and a venous phase) to maximise tumour characterization based on vascular contrast uptake and release [31]. The high spatial resolution of CT combined with the isotropic pixel size allows the image to be formatted in different planes to better define the tumour and adjacent vascular structures for segmental localization. For example, neuroendocrine carcinoma metastases are often highly visible on arterial phase imaging, while colorectal adenocarcinoma metastases are better appreciated in the delayed venous phase when the remainder of the liver parenchyma is contrast enhanced. Multidetector vertical CT scanners provide 1-2 mm images that can be reconstructed in coronal and sagittal projections to facilitate tumour localization [32].
Due to the availability of multiple image sequences and multiple contrast agents, such as T1-weighted imaging and diffusion-weighted imaging, MRI is particularly useful for identifying lesions that cannot be accurately characterised by CT. The portal vein phase (approximately 60-70 seconds after contrast administration) is the most reliable phase, with a CRLM detection rate of 85% and a positive predictive value of 96%. CRLM is hypo-vascular; arterial phase imaging does not improve prognosis but is useful for surgical and pre-embolic planning. CRLM < 10 mm is difficult to characterise on a CT scan. In addition, liver fat is not preserved after chemotherapy, which can further limit the detection of liver metastases [33]. MRI (Magnetic Resonance Imaging) has soft tissue contrast, making it an invaluable tool for detecting and characterising CRLM, especially those < 10 mm. CRLM is typically T1-hypodense, mildly T2-hyperintense, mainly ring-enhancing in the arterial phase, and hypo-enhancing in the portal vein and delayed phase [24].
DWI (diffusion-weighted imaging) measures the movement of water molecules in tissue. Apparently, the value of the diffusion coefficient is a quantitative estimate of the diffusion limitation. CRLM shows limited diffusion of water molecules due to high cellular intensity, resulting in high signal intensity with low apparent diffusion coefficient values. The addition of DWI increases the sensitivity and specificity for lesion detection and characterization.
HSCA (Hepatocyte Specific Contrast Agent) is very sensitive to small lesions that may be occult or have other consequences. It also allows for the discovery of "proliferative lesions" that may respond to emerging therapies. Gadobenate dimeglumine and gadoxetate disodium are both hepatocyte-specific contrast agents that are primarily taken up by hepatocytes and secreted into the biliary tree. Normal hepatocytes are hyperintense compared with liver metastases that do not retain contrast media. DWI has similar sensitivity and specificity to MRI and cellular contrast media but has lower sensitivity when performed with certain agents [34].
There is a lack of clinical evidence that PET (positron emission tomography), Fluor18 (8FDG PET-CT), has a significant impact on the clinical management of locally advanced metastatic colorectal cancer. Its role in the early diagnosis of colorectal cancer has not been determined. Most centres do not recommend routine PET-CT at this stage. However, it has been found to be overly accurate in detecting metastases, and this can lead to changes in the management of up to 1/3 of patients, avoiding the necessary metastasectomy with a significant impact on survival. There is sufficient evidence to support the use of PET-CT in routine follow-up, but if CT does not localise the disease, it may play an additional role in relation to elevated CEA levels. Two important caveats are the sensitivity of PET to detect tumour cells < 1 cm in diameter and its sensitivity to detect colorectal adenocarcinoma when patients are receiving chemotherapy.
SRS (somatostatin receptor scintigraphy) may be useful in identifying neuroendocrine carcinoma cells; however, it does not seem to increase the level of diagnostic information that can be provided by CT and MRI alone.
DOTATATE PET Gallium-68 imaging has been found to be highly sensitive and specific in the evaluation of neuroendocrine carcinoma [35]. Similarly, diagnostic laparoscopy and laparoscopic ultrasonography also help in the selection of patients ideal for resection [36].
Liver resection surgery revolves around the concept of FLR (future liver remnant), which is defined as part of the liver remaining after liver resection. FLR is usually capable of postoperative hypertrophy. However, FLR should be able to function at a minimal level to prevent postoperative liver failure [37].
FLR should be well vascularized; it must have adequate biliary drainage; it must have sufficient venous drainage; and it must have an adequate size. Using contrast-enhanced CT or MRI, the volume of the FLR and the volume of the entire liver can be estimated. Therefore, the ratio of the volume of FLR to the estimated volume of the liver (or, alternatively, if the total volume of the liver depends on the size of the patient) can be calculated. FLR-total liver volume-is the minimum required to maintain adequate liver function and hypertrophy, depending on the condition of the liver.
In patients with normal liver function, this ratio can reach 20%. In severely decompensated patients (for example, as a result of cirrhosis), the minimum FLR ratio can be 50%. Prolonged chemotherapy-induced hepatotoxicity may require a minimum FLR of 40%. In advanced disease with large or multifocal tumours, operative planning may be necessary to maintain an adequate FLR. A parenchymal-sparing approach to liver metastasectomy can use adjuvants such as thermal ablation or portal vein embolisation [38].
FLR was calculated as the fraction of FLR (ml) and Total Functional Liver, the latter being the difference between Total Liver Volume (TLV) and Tumour Volume (TuV). Liver resection is considered technically feasible and oncologically feasible, but the amount of remaining liver is insufficient (i.e., 25% of the total liver volume). In such cases, methods for developing FLR should be considered. FLR development options include portal venous embolisation, portal venous ligation and combined hepatic resection, and portal venous ligation for staged hepatectomy. However, FLR volume does not necessarily reflect performance, especially since the quality of liver tissue can be affected by preoperative chemotherapy.
Indocyanine Green (ICG) is a tricarbocyanine dye that binds to albumin and distributes evenly into the blood within minutes after intravenous injection. ICG is taken up by the liver and excreted in bile without any conjugation. There are some limitations to the use of ICG. Hepatocyte uptake may be impaired by hyperbilirubinemia, and it reflects overall liver function instead of FLR performance without resolving changes in the liver. The amount of ICG remains in the blood for a certain time after the injection for the stratification of liver resection. The goal of liver metastasectomy is complete resection with negative resection margins. However, the safety of liver surgery has improved significantly in recent decades, with operative mortality after liver resection around 1-3% in large centres. The main risks are bleeding, gallstones, and the most devastating complication that can occur after liver surgery, postoperative liver failure [39].
Makuuchi's criteria were established in the 1980s as a method to predict safe liver resection in patients with HCC and liver failure [40]. However, with detailed risk assessment and 3-D volume surgical planning, an individualised approach is now feasible. The expanded Makuuchi's dimension and the possibility of extending the ICG-K and 3-D liver volume measurements have been explored.
In addition, Makuuchi, et al. first proposed the use of portal vein embolisation (PVE) as a strategy to promote hypertrophy in hepatocellular carcinoma patients showing FLR deficiency. PVE promotes FLR development by diverting portal flow to the FLR and promoting hypertrophy through hepatocyte clonal expansion and increased liver growth factor production [41].
The most basic strategy involves the crush-clamp technique, where the surgeon divides liver parenchyma with his fingers. This can be further enhanced by employing the basic surgical clamps to crush the hepatic parenchyma. This technique is simple, quick, efficient, easy to learn and perform, and cost-effective.
The cavitron ultrasonic surgical aspirator is a better method for surgical resection. Advantages include the fact that CUSA is able to provide a very well-defined plane, which is useful in situations of close proximity between tumours and major vasculature structures. Also, it can be used in cirrhotic as well as non-cirrhotic livers and is associated with low blood loss and a low risk of bile leak. The limitations are that CUSA is able to dissect tissue but doesn’t offer coagulation or hemostasis.
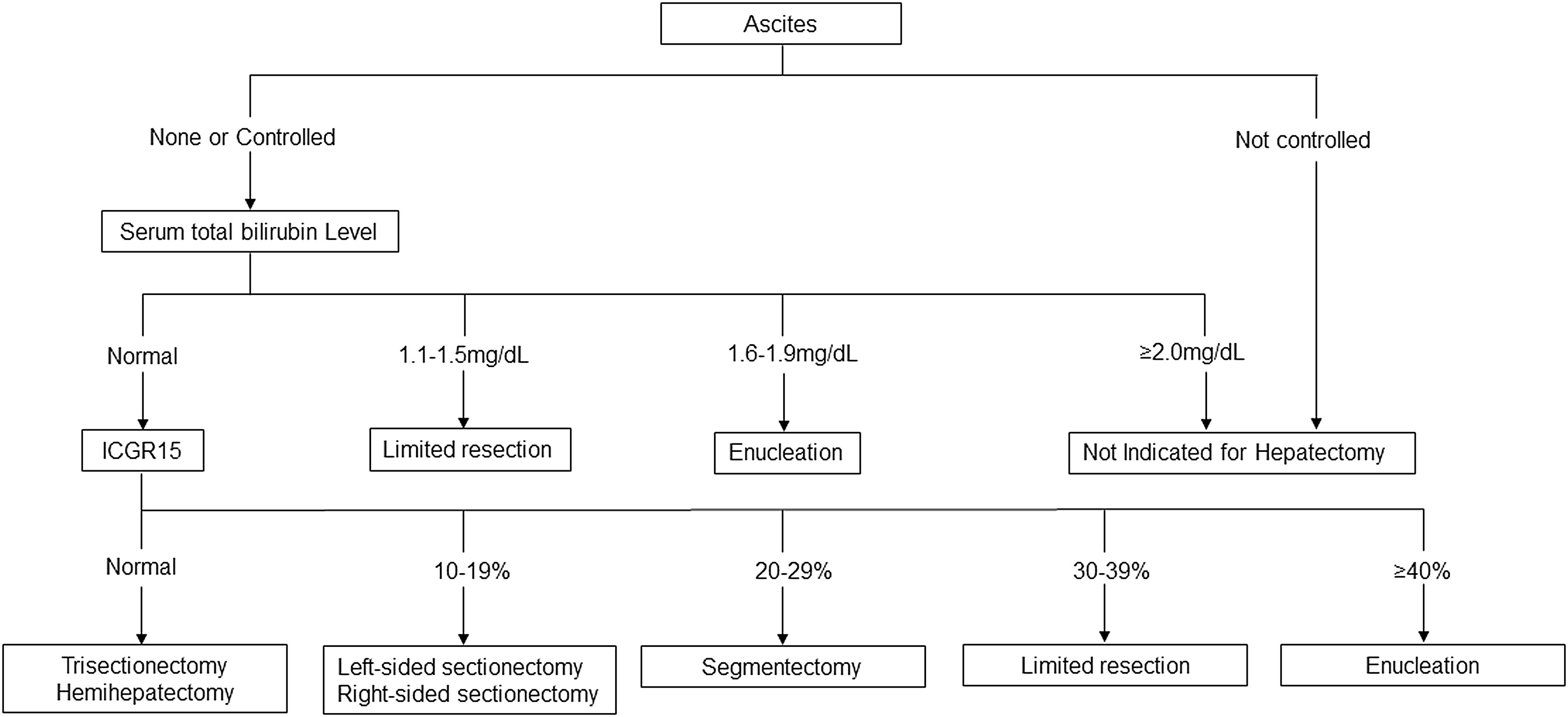 Chart 1:
View Chart 1
Chart 1:
View Chart 1
The LVSS (Ligasure Vessel Sealing System) is a bipolar vessel sealing system that can permanently occlude blood vessels up to 7 mm in diameter by combining pressure and energy to fuse the collagen matrix in the vessel [42].
The salient dissecting sealer achieves blunt parenchymal dissection and hemostatic sealing of small vessels at the liver surface. This technique is also available for laparoscopic application, especially for cirrhotic livers and in obtaining adequate surgical margins when metastatic colorectal tumours are located near major biliary structures due to its ablative nature.
Radiofrequency-assisted liver resection technique is also another modality employed for resection, but the only disadvantage is that due to the excessive charring, there is a potential risk of infection at the surgical sites [43].
A common treatment algorithm for patients with advanced multifocal disease is a two-stage hepatectomy. In this approach, two separate surgical procedures are used to completely remove the liver tumour. In the first stage, surgical resection and ablation are used to clear the expected FLR. This is often done minimally invasively to facilitate recovery and the transition to the next treatment. Shortly after this procedure, a contrast-enhanced PVE is performed to detect hypertrophy of the newly resected part of the liver. If post-PVE imaging verifies satisfactory hypertrophy of the FLR, hepatectomy is completed to remove residual disease [44].
Liver transplantation is the only treatment option for patients with liver disease who cannot recover from neuroendocrine tumours. Mazzaferro, et al., in 2007, used the criteria of Milan, Pavel, et al., in 2012, in partnership with the guidelines of the European Neuroendocrine Tumour Society (ENETS), to make the first attempt to determine the selection criteria for liver transplantation in such patients. According to this study, the selection criteria for liver transplantation are: liver involvement less than 50% parenchyma; no severe disease; well-differentiated neuroendocrine tumours (Ki-67 proliferative index less than 10%); age < 55 years; first tumour resected at least 6 months before transplantation with current stable liver disease; and the location of the primary tumour [45].
There are several potential benefits of CTX in preoperative patients with advanced disease, such as limiting disease progression, limiting hepatectomy, treating micro-metastases, and evaluating the chemosensitivity of the disease that CTX should be given after resection of metastases. One major concern with neoadjuvant CT is the development of metastases during neoadjuvant CT. Preoperative CTX administration has been shown to increase postoperative morbidity and mortality. The aim of consecutive CTX after resection is to prevent recurrence in the remaining liver and to treat additional occult liver metastases. Surgery enables tumour resection and allows CTX to target micrometastatic disease. Until 2000, the standard treatment for CRLM was based on palliative CT using 5FU (or fluoropyrimidine) drugs combined with folinic acid (LV) in about 20% of cases. However, the administration of a combination of 5FU/LV, irinotecan, and oxaliplatin (FOLFIRINOX) has been developed to improve treatment outcomes and increase the proportion of patients exposed to all active agents [46].
Currently, two molecules are included in the first-line treatment of metastatic CRC. Bevacizumab, a humanised monoclonal antibody that targets a key factor in tumour angiogenesis called VEGF (Vascular Endothelial Growth Factor), is the first molecule developed in the treatment of CRLM with proven benefits. Cetuximab, a chemical monoclonal antibody that targets the EGFR (epidermal growth factor receptor), showed improvement in both patients over 24 months [47].
The purpose of HAI (Hepatic Arterial Infusion) is to deliver therapeutic drugs directly to the liver and obtain high drug concentrations in the tumour. This technique is often used in palliative situations where the liver is unsuitable for surgical resection. Advantages of this method show that with the strategy of combining HAIFUDR (5-fluoro-2-deoxyuridine) and IV drugs (irinotecan, 5-FU, oxaliplatin, and irinotecan), an increase in the tumour response rate of up to 80% can be achieved. In selected patients, this combination resulted in 80% as first-line therapy and 50% as second-line therapy. The disadvantage is that technical difficulties for catheter placement have limited the implementation of this technique in routine practise [48].
A technique for injecting chemotherapy into the artery that feeds the tumour is to use a particle designed to slow or stop the supply of oxygen and nutrients through the artery to the tumour. Arterial chemoembolization can also be called TACE (transarterial chemoembolization) and is the most effective treatment in the spectrum of palliative care.
TACE kills cancer cells through high concentrations of cytotoxic agents and ischemia. Mitomycin C and cisplatin/doxorubicin-based conventional TACE salvage therapy offered a median survival of 14 and 11 months from initiation, respectively. The new drug-eluting bead (DEB)-TACE uses microspheres filled with cytotoxic drugs (doxorubicin or irinotecan). The drug is released in a controlled manner, and high intratumor doses can be delivered while low plasma concentrations can improve patient tolerance. Concomitant administration of capecitabine improved disease control but not survival; adding FOLFOX and bevacizumab increased the rate of conversion to resection. In nonsurgical candidates, RFA combined with TACE achieved local tumour control in 92%, and OS at 2 years was 88%.
DEBIRI (Drug Eluting Beads Using Irinotecan) consists of trans-arterial delivery of irinotecan-coated beads, which theoretically allows slow drug delivery for prolonged antineoplastic effects. Limitations: The role of TACE in the treatment of CRLM is limited, although this treatment is more designed to slow disease progression in HCC. Fewer patients (30%) experienced drug-related side effects compared with TACE, and most had only minor symptoms. abdominal pain, vomiting, and fever. Postembolization syndrome, consisting of nausea, fever, fatigue, and liver tenderness, has been reported in 2/3 of patients, but most include liver abscess, liver failure, and peptic ulcer disease [49].
This technique requires administration of therapeutic agents through the hepatic artery and aspiration of hemosaturated blood from the inferior vena cava. This is usually done during open surgery, but can be achieved through a percutaneous approach with interventional radiology. In the catheter technique, a catheter is inserted into the hepatic artery or IVC to perform perfusion and also to draw hemosaturated blood, which is filtered and returned to the patient. This procedure should be performed with hemodynamic monitoring and support, as it requires invasive vascular access as well as close care of critical blood vessels. The first described therapeutic agents used in IHP are tumour necrosis factor and melphalan, which can show an overall objective radiographic response rate of 60-70%. Recently, oxaliplatin has been triangulated as a representative of IHP [50].
It is suitable for patients with tumour recurrence after previous liver resections. In such cases, tumour growth can occur with ablation in patients tired of long-term systemic chemotherapy [51]. In radiofrequency ablation, a modified electrocautery technique is used, and the function of a probe is to pass an alternating high-frequency current through the tissue. At 50-52 degrees Celsius, cells undergo coagulative necrosis in 4-6 minutes. New research shows it is possible to remove a 7-cm-diameter tumour in 30 minutes. Depending on the location and number of tumours, RFA can be performed laparoscopically or through an open percutaneous technique under radiological guidance. The use of laparoscopic and open surgical procedures for therapeutic guidance allows better localization of lesions and methods of monitoring ablation progress. Microwave ablation uses an electromagnetic field to remove heat directly from the tumour, and microwave fields can heat larger tissues more quickly. In addition, there is little effect from regional heat transfer from adjacent vessels. This can allow for larger, more predictable network releases at higher speeds. However, reports on MVA remain limited. Cryoablation involves inserting a cryoprobe through the liver parenchyma into the liver embryo. Lesions are destroyed by freezing to -190°C followed by thawing. Detection and removal of lesions were monitored by intraoperative ultrasonography. Recent advances in laparoscopic ultrasonography and cryosurgery have allowed this procedure to be performed completely laparoscopically. Laparoscopic ablation of liver tumours is an emerging technology in the field of minimally invasive surgery. Liver metastases can be removed by several methods, one of which is percutaneous ethanol injection. A special needle is inserted into the tumour, and then an alcohol injection is used to kill the cancer. Ultrasound and computed tomography are imaging procedures. Alcohol destroys tumours by removing water from tumour cells (dehydrating them) and changing the protein structure of cells (denaturing) [52].
In addition to these methods, unresectable CRLM can be treated with radiation-assisted selective intraoperative radiotherapy, stereotactic body radiotherapy, and palliative whole-liver radiotherapy. Intraoperative radiotherapy is a targeted approach that delivers yttrium-90-labelled glass or resin microspheres, mainly beta particle emitters, to the arterial portion of the metastatic or primary liver tumour. As a basis for the potential selection of techniques, post-infusion histological analysis showed that the spheres were secreted by a small amount of normal liver into the vessels around the tumour. Stereotactic body radiation therapy is a non-invasive method to provide local treatment for liver disease with ablative intent. This technique uses a high dose (6-10 Gy) per day delivered over 3-5 fractions, resulting in an effective biological dose. Patients are stimulated with various techniques to reduce tumour motion, including breathing and abdominal compression, and highly compliant radiation plans are designed to deliver extremely high doses beyond the planned target volume (PTV) and normal tissue. In general, SBRT can deliver more intense radiation to individual discrete lesions, while SIRT can be used for larger and more widespread liver involvement [53]. Palliative radiation therapy based on the whole liver, as the name suggests, is more appropriate when the goal of oligometastatic disease is to move away from radical, potentially curative ablation to palliate symptoms in patients with life-limiting disease. An enlarged liver can sometimes cause nausea, anorexia, and pain due to enlargement of the liver capsule, among other symptoms. A reduction in patient-reported symptoms occurred in most patients. Nearly 55% experienced at least partial relief of abdominal pain, 49% experienced nausea and vomiting, and 28% experienced less anorexia [54].
This phenomenon is described as the complete disappearance of liver metastases on cross-sectional imaging such as CT, PET, or MRI, also known as the complete radiological response. Described in 37% of patients after neoadjuvant systemic therapy, However, local residual disease at the site of missed metastases can be found in 11-67% of patients at laparotomy. In addition, there is a clear difference between a complete radiological response and a complete pathological response. When resected liver metastases are excised, microscopic residual disease is found in up to 80% of specimens [55].
A skilled multidisciplinary team consisting of at least one colorectal surgeon, one hepatobiliary surgeon, a medical and radiation oncologist, a radiologist, and a pathologist optimises care. Treatment should be considered holistic, from diagnosis to final treatment at the centre. Evaluating the results of MDT to assess improvement in treatment goals Routine pathologic evaluation should include size, number, margin, toxicity to non-tumour cells, micro-metastases, and the malignant halo.
Reliable resection margin: Still a goal, a minimum margin of 1 mm is recommended. Tumour response to prior chemotherapy as assessed by TRG and/or viable tumour cells. There are no established biological markers that can differentiate the biology and prognosis of synchronous and metachronous liver metastases. Molecular assessment of liver metastasis plays an increasingly important role in evaluating the biology of colorectal liver metastasis. And the concept of minimally invasive surgery for the resection of primary colorectal cancer and liver metastasis was also given for doing both surgeries in single settings by postulating different port positions in such patients.
General Principles of Symptom Control include focusing on the quality of life in the present and not on death.Potential for adaptation, integration, reconciliation, and transcendence including psychological, psychosocial & spiritual care.
We are grateful for the contribution of each author in the present review article. Dr. Aditya Sharma is the primary author who drafted this manuscript, and the co-authors Prof. Ram Niwas Meena, Prof. Rahul Khanna & Prof. Seema Khanna checked and made substantial contributions to the conception, design of the work and the acquisition, analysis, or interpretation of data for the work and approved the final manuscript for publication.
1. Dr. Aditya Sharma: Drafted the manuscript and submitted the manuscript under the supervision of Prof. Ram Niwas Meena, Prof. Rahul Khanna & Prof. Seema Khanna.
2. Prof. Ram Niwas Meena: Checked and made substantial contributions to the conception or design of the work and the acquisition, analysis, or interpretation of data for the work and approved the final manuscript for publication.
3. Prof. Rahul Khanna: Checked and made substantial contributions to the conception or design of the work and the acquisition, analysis, or interpretation of data for the work and approved the final manuscript for publication.
4. Prof. Seema Khanna: Checked and made substantial contributions to the conception or design of the work and the acquisition, analysis, or interpretation of data for the work and approved the final manuscript for publication.
Nil.
Human subjects: Consent was obtained from or waived by all participants in this study.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment and service information: All authors have declared that no financial support was received from any organisation for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organisations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
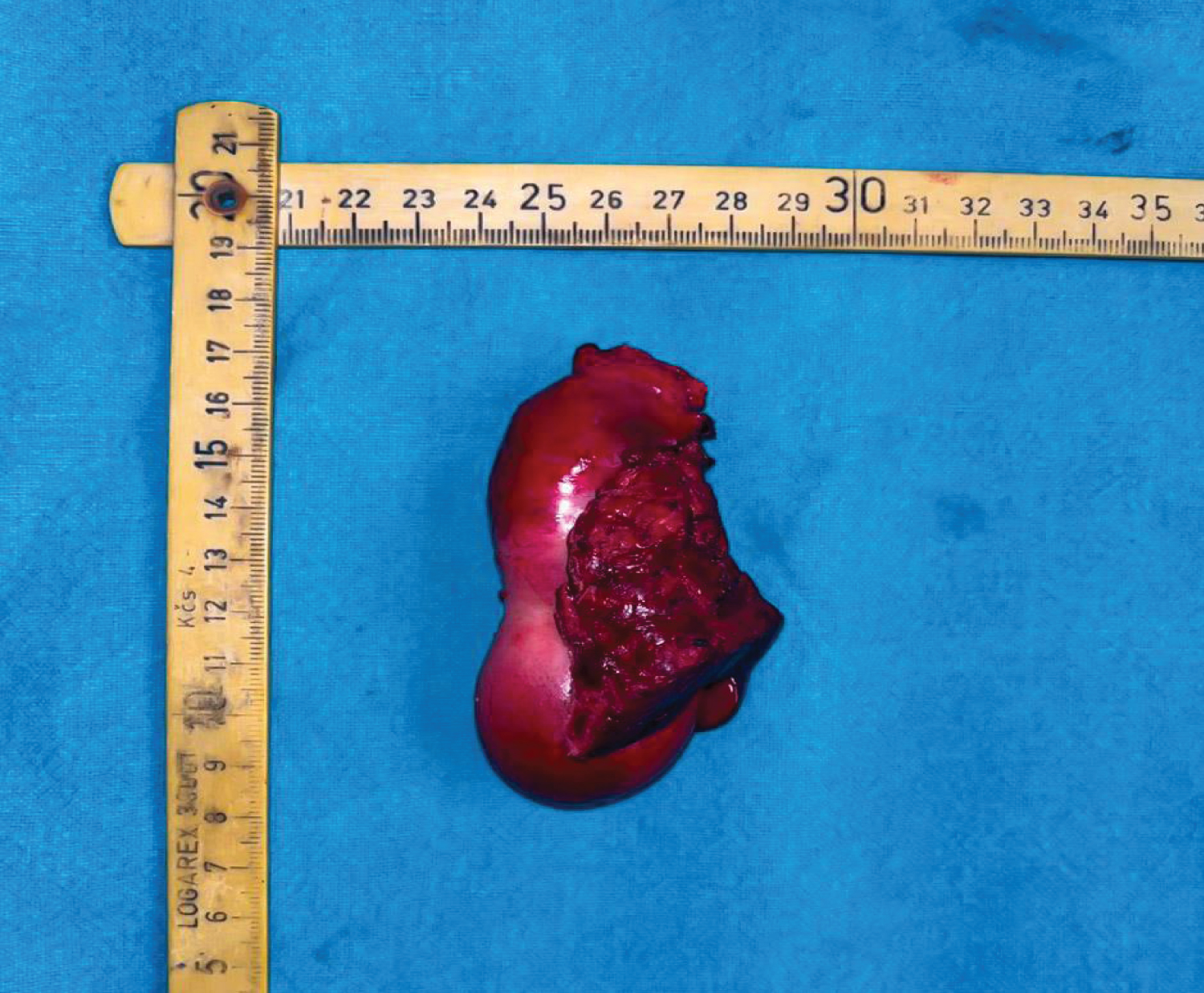 Figure 1: A resected specimen of Carcinoma Gall Bladder along with the portion of infiltrated Segment IV & Segment V.
View Figure 1
Figure 1: A resected specimen of Carcinoma Gall Bladder along with the portion of infiltrated Segment IV & Segment V.
View Figure 1
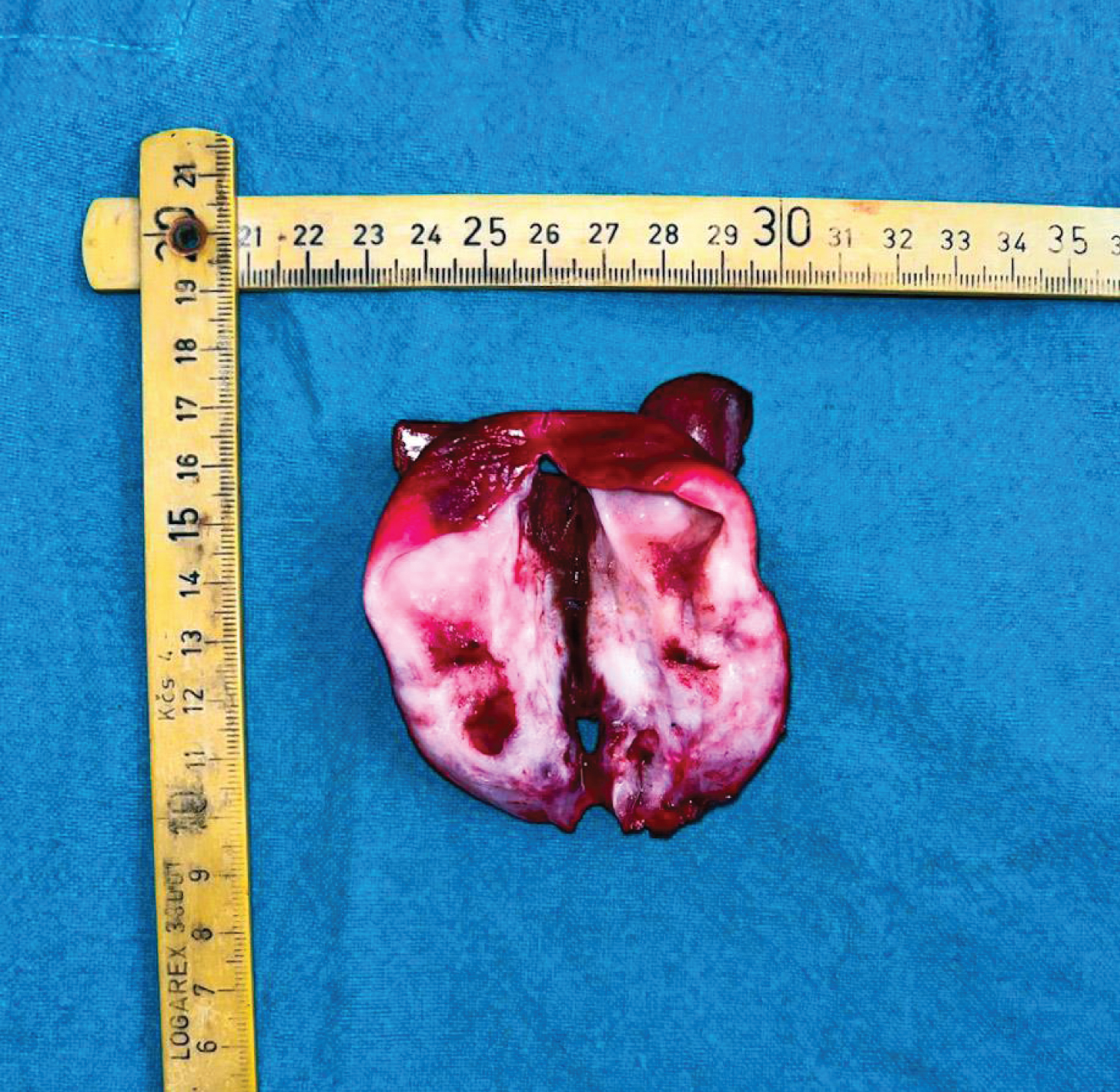 Figure 2: A cut specimen of the Gall Bladder showing irregular thickening of the wall of gall bladder.
View Figure 2
Figure 2: A cut specimen of the Gall Bladder showing irregular thickening of the wall of gall bladder.
View Figure 2