Ludwig's angina (LA) is a clinical diagnosis characterized by a rapidly spreading infection involving the floor of the mouth and surrounding submandibular, sublingual and submental spaces [1]. Early recognition and prompt treatment of LA is critical as untreated LA has a myriad of complications, most commonly airway obstruction with a subsequent mortality rate of 50% [2]. Odontogenic etiology is implicated in 70-90% of cases in the adult population [3], reasoning lies in the fact that the roots of the second and third molars penetrate the mylohyoid ridge creating a direct avenue for odontogenic infection to invade the submandibular space. Infection can then spread continuously to the sublingual space, pharyngomaxillary, and retropharyngeal space creating a tenuous airway [4]. Other etiologies include orofacial trauma, sialadenitis, and tongue/oral piercings. Immunodeficiency, diabetes mellitus, chronic alcohol use and injection drug use have all been found to be risk factors for LA [2]. Common causative organisms are Viridans group streptococci, Staphylococcus aureus, and Staphylococcus epidermidis accounting for over 90% of cases [2,4]. Patients usually present with systemic and localized symptoms such as fever, malaise, chills, generalized weakness, odynophagia, dysphagia, trismus, dysphonia (‘hot potato voice’), and drooling [3]. Physical exam will reveal a tender, woody, and indurated submandibular area. The lingual area may also be erythematous and swollen. The tongue may be displaced posteriorly secondary to inflammation and the outer neck may be tender and edematous with or without focal lymphadenopathy [2]. Although imaging is not necessary for diagnosis, common findings on computed tomography (CT) demonstrate diffuse soft tissue swelling and fat stranding of the submandibular, sublingual, submental, and cervical areas, evidence of soft tissue gas, fluid collection, and muscle edema [2,5]. Management of LA can include surgical intervention, antibiotics, and strict airway monitoring. Despite advancements in treatment, the mortality rate of LA is 8% [4]. Herein, we present a case of a middle-aged woman with LA caused by Enterobacter cloacae in the setting of pre-existing tracheal stenosis.
FB is a 57-year-old female with multiple comorbid medical conditions including tracheal stenosis status-post multiple dilations, coronary artery disease, hypertension, and end-stage renal disease (ESRD) receiving hemodialysis who presented with a 4-day history of bilateral odynophagia with associated right-sided neck swelling and new onset fever, sore throat, shortness of breath, and bilateral neck swelling. The patient also reported associated chills, nonproductive cough, nausea, and one episode of nonbilious, non-bloody emesis. She was able to tolerate oral intake at this time and presented without drooling or other signs of impending airway obstruction. The patient was febrile at presentation with a temperature of 38.7 °C. CT was obtained which demonstrated a multiloculated fluid collection within the floor of the mouth suspicious for an abscess and significant tracheal stenosis at the level of the thyroid gland measuring 8 × 6 mm. The collection of fluid at the base of the tongue had its largest pocket measure 2.2 × 1.4 cm (Figure 1). Blood cultures were obtained at the time of her presentation, and she began receiving empiric intravenous (IV) vancomycin and metronidazole. IV dexamethasone 10 mg q6 hours was also administered at this time. The patient was subsequently admitted to the medical intensive care unit for airway monitoring and continuous pulse oximetry. Blood cultures revealed Staphylococcus epidermidis on hospital day 3 and her antibiotic regimen was changed to IV vancomycin and ampicillin-sulbactam per our infectious disease team recommendations. She was deemed stable by our otolaryngology team to be transferred to the hospital floor on hospital day 4, however, the patient began reporting increased stridor, dysphonia, pain, and neck swelling on hospital day 5. Repeat CT demonstrated an increase in the fluid collection in the right floor of the mouth which now measured 3.5 × 2 cm (Figure 2). The patient was a febrile at this time, but was being closely monitored for acute decompensation. Surgical planning for incision and drainage of the growing floor of mouth abscess was made and the patient was boarded for surgery. A direct laryngoscopy was performed to visualize and drain the left low oropharyngeal/glossotonsillar sulcus and infected cystic lesion. A large amount of purulence was drained and sent for culture. She also was found to have a small to medium sized vallecular cyst which was biopsied and sent for pathology with no associated purulence. She tolerated the procedure well and seemed to be improving clinically with reduced neck swelling and improved dysphagia. Her steroids were slowly tapered post-procedure. Surgical pathology of the biopsied vallecular cyst resulted in benign squamous mucosa with a lymphocytic infiltrate. Final bacterial cultures from the oropharyngeal abscess grew Enterobacter cloacae resistant to ertapenem, few Candida tropicalis , few Streptococcus viridans , and rare Lactobacillus species. Her antibiotic regimen was subsequently switched to oral meropenem 500 mg q 24h for Enterobacter coverage and oral fluconazole 200 mg QD for candida coverage. She was subsequently discharged home on oral fluconazole, sulfamethoxazole/trimethoprim, and metronidazole with home health care. She will continue to follow-up with ENT for her subglottic stenosis.
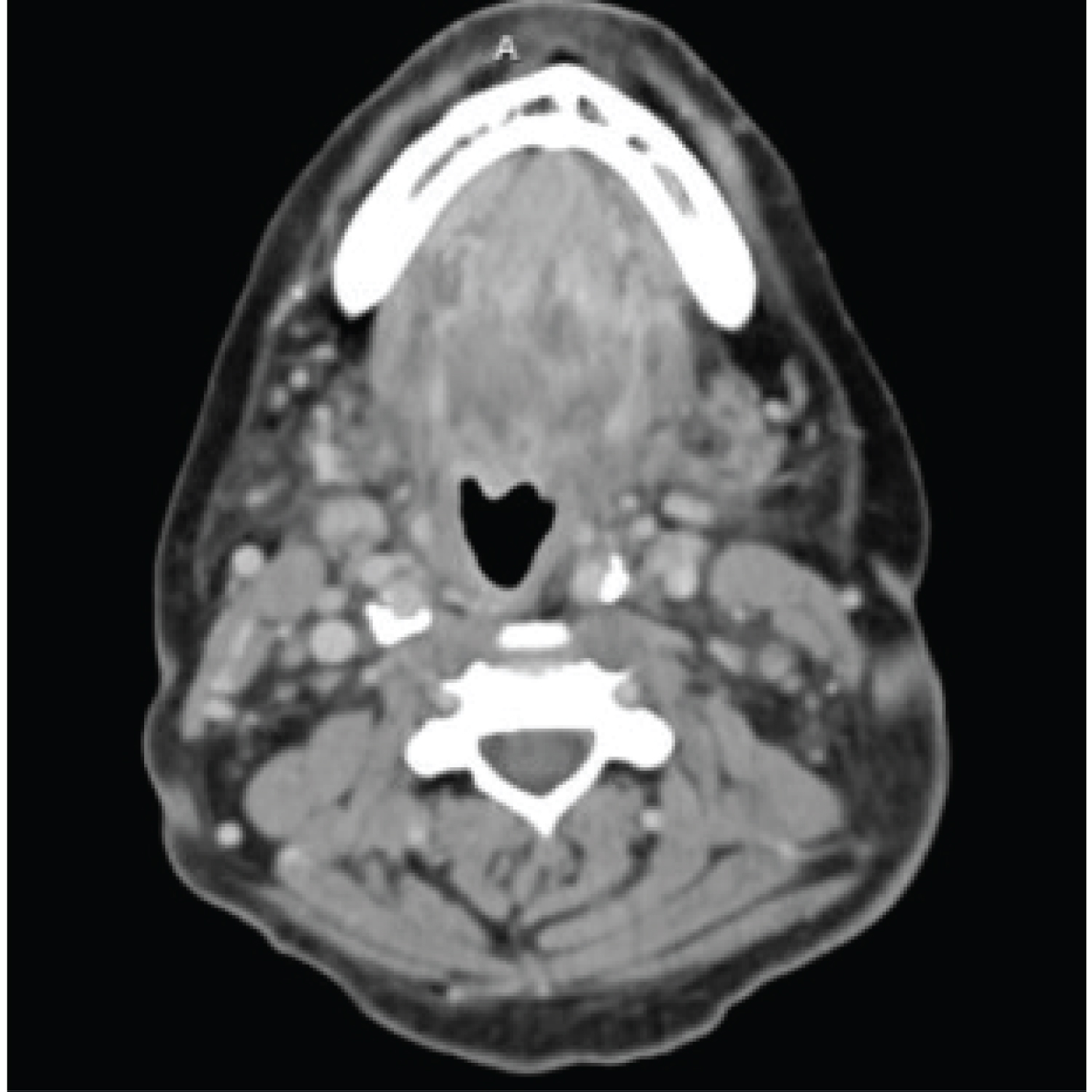 Figure 1: The collection of fluid at the base of the tongue had its largest pocket measure 2.2 × 1.4 cm.
View Figure 1
Figure 1: The collection of fluid at the base of the tongue had its largest pocket measure 2.2 × 1.4 cm.
View Figure 1
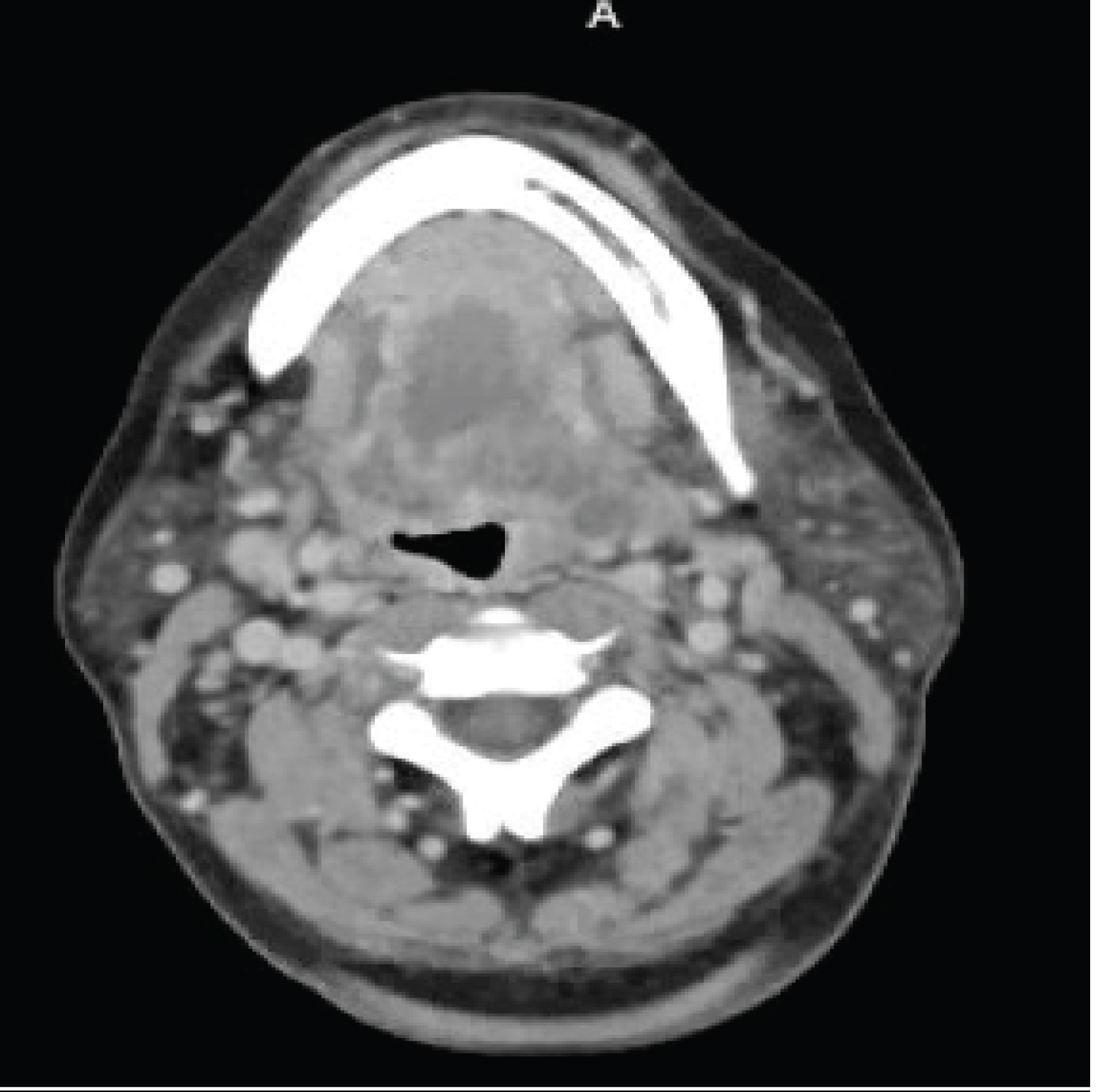 Figure 2: Repeat CT demonstrated an increase in the fluid collection in the right floor of the mouth which now measured 3.5 × 2 cm.
View Figure 2
Figure 2: Repeat CT demonstrated an increase in the fluid collection in the right floor of the mouth which now measured 3.5 × 2 cm.
View Figure 2
The current case describes a middle-aged woman with multiple comorbidities and significant otolaryngologic history who underwent a complicated hospital course secondary to LA. After eventual incision and drainage of the abscess, we were able to obtain accurate bacterial cultures demonstrating infection with Enterobacter cloacae . E. cloacae is a gram-negative, rod-shaped, facultative anaerobe that normally flourishes in the microflora of the intestinal tracts of humans and with no known implication as a causative organism in LA [6]. Enterobacter infections have a high mortality rate; one study demonstrated that Enterobacter bacteremia carried a 24.6% 30-day mortality rate in patients receiving appropriate antibiotic therapy [7]. Similar mortality rates have been reported for soft tissue infections due to Enterobacter [8]. E. Cloacae’s high mortality rate is partially attributed to its many virulence factors including enterotoxins, alpha-hemolysin, thiol-activated pore forming cytotoxins that function similarly to Shiga-like toxin II, and a type III secretion system [9].
An infection with E. cloacae is challenging to treat as it carries significant antibiotic resistance; the pathogen has two separate constitutive beta-lactamases, AmpC B-lactamase, and extended-spectrum B-lactamase (ESBL), that are not inhibited by common inhibitors of B-lactam [6]. Due to B-lactamase activity, E. Cloacae is resistant to ampicillin, amoxicillin, first generation cephalosporins and cefoxitin. Moreover, treatment with a third-generation cephalosporin may select for strains that overproduce Amp B-lactamase, worsening antibiotic resistance [6]. Therefore, the mainstay of treatment of E. Cloacae infections are carbapenems, fluoroquinolones, aminoglycosides, or sulfamethoxazole/trimethoprim [8]. Of important mention, there have been several reports of emerging carbapenem-resistant strains of Enterobacter, implicating an even greater need to identify organisms on culture as soon as possible and to treat them appropriately [9].
In the current case, the patient’s blood cultures revealed Staphylococcus epidermidis , which is a common causative organism in LA, however in hindsight we were grossly misled and the cultures were likely contaminated by skin flora. Common empiric treatments for LA in immunocompetent patients include ampicillin-sulbactam, ceftriaxone, metronidazole, clindamycin or levofloxacin [2], which were trialed in the current case without improvement. It was not until the patient’s abscess was able to be surgically incised, drained, and cultured that we were able to unveil E. cloacae , the pathogen at hand. This further supports the notion that LA patients should be considered for surgical intervention, especially in those who do not respond to medical management.
E. cloacae has been found to be a major source of nosocomial infections contributing to skin and soft tissue infections in addition to bacteremia, endocarditis, septic arthritis and osteomyelitis [6,10]. Additionally, this pathogen has been found to contaminate various medical, intravenous, and hospital devices [11]. There have been few studies that implicate this pathogen in head and neck infections, however, Ramos-Zayas, et al. frequently identified E. cloacae in postoperative infections following head and neck free flap reconstruction surgeries [12]. Additionally, there has been one case report describing an infection with E. cloacae of the nasal septum after undergoing septoplasty [13]. The authors in that case administered ciprofloxacin after E. cloacae was identified but resulted in subsequent anterior septum perforation.
Although the etiology of LA is variable, the majority of cases in adults arise in those with poor dentition with resultant oral infection and extension into the floor of the mouth [2]. In the current case, oral surgery was consulted to evaluate for any indication of odontogenic origin of the abscess. Even though the patient had an extraction planned for her upper left maxillary incisor with her general dentist, she denied any other dental pain. Oral surgery reported that the mandibular teeth were intact without any signs of infection, effectively ruling out an odontogenic source. The patient in the current case has a significant medical history of ESRD requiring hemodialysis three times weekly and given E. cloacae ’s rampant association with healthcare environments and medical devices, this creates ample opportunity for the patient to be exposed to E. cloacae . Furthermore, the patient has an extensive otolaryngology history including tracheostomy placement and removal with resultant tracheal stenosis. She underwent tracheal resection as well as numerous tracheal dilations and procedures to treat her stenosis. We hypothesize that the repeated manipulation and trauma may have impaired the mucosal barrier as well as other local immune defenses creating a nidus for infection. The current case demonstrates why vigilance is necessary when managing LA and to consider unusual pathogens when mainstay treatment fails.