Rehabilitation of edentulous ridges with implants is becoming so popular. However, it becomes onerous for a dentist to place implants in severely resorbed ridges which requires tension free primary wound closure. Instead of using invasive and extremely tedious methods to augment soft tissue, an intra-oral soft tissue expander is an ideal option.
This review aims to pool all the data available on the intra-oral soft tissue expander and all the recent advances of the concept of soft tissue expansion.
The electronic data bases search on pubmed, google scholar and cochrane database for articles searched until June 2020.
Soft tissue expander can be used as a very effective treatment option for increasing the soft tissue volume before ridge augmentation. There are insufficient human intra-oral studies validating this method. However, there is a need for more well-designed human trials to comprehend its use.
Tissue expanders, Soft tissue expander, Osmed® expanders, Hydrogel expanders, Intra-oral soft tissue expander
Soft tissue complications like wound dehiscence after ridge augmentation is around 16.8% which occurs mostly due to inadequate tension free primary would closure [1]. Similarly, Proussaefs & Lozada reported 25% wound dehiscence in patients with vertical bone augmentation with autogenous bone blocks [2]. Inadequate tension free primary closure is due to insufficient amount of overlying soft tissue. One way to avoid wound dehiscence after guided bone regeneration technique is by using vertical releasing incisions and periosteal releasing incision for flap advancement. However these two incisions greatly compromises vascularisation of the surgical area since it disrupts the continuity of the flap and the periosteum [3,4]. Decreased vascularisation and wound dehiscence negatively affect the outcome of ridge augmentation [3]. For primary wound closure and to prevent post-operative complications, a very successful soft tissue management is mandatory. Free fibula flaps [5], distraction osteogenesis [6], periosteal distraction [7] and tissue engineered periosteum [8] are some available methods for soft tissue augmentation but their use for long length edentulous ridges is very difficult. In plastic and reconstructive surgery the soft tissue augmentation is done with the use of tissue expanders [9]. This idea was mimicked with the use of intra-oral soft tissue expanders to improve the overlying mucosal volume before ridge augmentation. Intra-oral soft tissue expander is a device that is placed in the sub/supra-periosteal pouch that expands gradually leading to increase in the soft tissue volume and thickness by mechano-transduction. This literature review primarily focusses on the use of intra-oral soft tissue expanders for soft tissue expansion in ridge augmentation.
The electronic data bases, pubmed, google scholar and cochrane library were searched for articles published from January 1950 until 1st June 2020.
The terms "Tissue expander", "Soft-tissue expander", "dental expander", "oral expander", "dental soft tissue expanders", "oral soft tissue expanders", "Hydrogel expanders", "Osmed® expanders" and "Osmed® soft tissue expanders" were used. Only human studies that have used intra-oral expanders for soft tissue expansion in ridge augmentation was included in the study. Excluded studies include the use of intra-oral expanders used in cranio-facial reconstruction and cleft lip and palate. Animal studies, literature and systematic reviews, in-vitro studies were excluded. The methodology of study selection is illustrated in Figure 1.
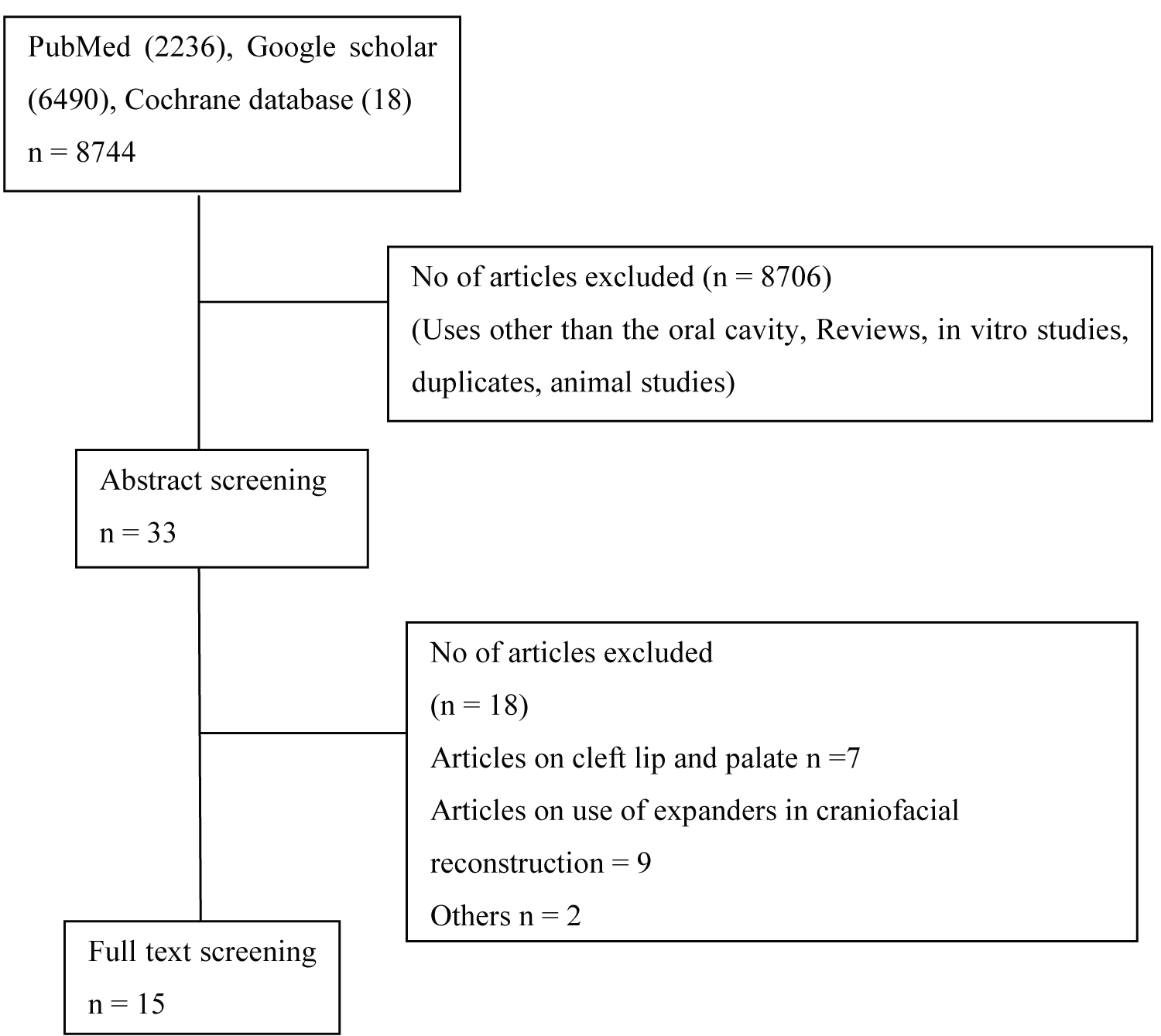 Figure 1: The methodology of study selection.
View Figure 1
Figure 1: The methodology of study selection.
View Figure 1
The methodological characteristics of the selected papers were assessed according to the PICO format i.e.
P (patient/population): Patients requiring alveolar ridge augmentation
I (intervention): Placement of soft tissue expander
C (comparison): Soft tissue volume with and without placement of expander
O (outcome): Soft tissue expansion
All the studies were assessed separately by both the authors and divergent opinions and assessments with regards to the assignment of strengths and weaknesses, consensus was reached by discussion.
The idea of using a tissue expander stems in 1957 when Charles Neumann was successful in reconstructing an external ear by placing an inflatable silicon rubber balloon beneath the skin and inflating it by multiple injections manually several times via an external port [10]. However due to presence of the port close to the reservoir, chances of infections and difficulty in tracing the port for injection made the procedure strenuous for the clinician. Chedomir Radovan constructed a conventional expander with inflatable reservoir but with self-sealing valve and external port for breast reconstruction [11,12]. The following year, Argenta used this technique for treatment of burns [13]. In 1992, Van Damme used a conventional expander in craniofacial defect [14]. Eric Austad and Rose developed a self-inflating hydrogen expander covered with semi-permeable silicon cover filled with hypertonic NaCl which did not require external port and multiple injections for inflation which overcame most of the trouble and cost of the treatment for the patient [15]. It was based on the principle that the expander would gradually swell due to the osmotic forces of the surrounding fluid into the hypertonic sodium chloride filled expander [15]. Hypertonic sodium chloride solution was used since the solute had to be a highly water-soluble, low-molecular weight, non-toxic compound that produces two ions in solution that double the effect of a single, non-ionised species (e.g.: Glucose) [15]. These expanders were custom fabricated by Dow-Corning Medical products in 1976. Later in 1981, it was used in clinical trials in two patients after multiple experimental studies [15]. However, it was observed that the hypertonic NaCl solution in the expander would leak out resulting in tissue necrosis [16].
As it is rightly said that "Innovation is a continuous process". To overcome the limitations of the previous expander, Wiese used a cross-linked hydrogel of co-polymers based on methyl methacrylate and N-vinyl-pyrrolidone. [17]. Osmed® (Ilmenau, Germany) designed and manufactured the first commercially available self-inflatable osmotic expander in 1999 but FDA approved it in 2001 [16]. Osmed® osmotic expanders were later available in two generations. First and second generation expanders only differed in the semipermeable silicon coating over the hydrogel present in the latter [16]. In 2007, the availability of intra-oral miniature size self-inflating expander made its use more easy and effective for soft tissue mucosal augmentation [16].
Tissue expanders can be mainly classified in two types:-
1) Conventional soft tissue expander:
The conventional tissue expanders were made of silicone rubber, with an external valve penetrating the skin for manual inflation by serial injections. Repetitive inflations increased the treatment time up to several months [10].
The method of expansion was intermittent, creating pressure peaks [18] that lead to hypoxia of the tissue leading to expander perforation [17,19]. Multiple injections for inflation of reservoir were cumbersome for the patient as well as for the clinician which also added the cost of the treatment and was accompanied by complications such as infections, tissue necrosis, tissue perforation and treatment failures [16].
2) Self-inflating soft tissue expander:
Self-inflating soft tissue expander consists of hydrogel encompassed with semipermeable silicon membrane without an external port [15]. Hydrogel is a three-dimensional (3D) network of hydrophilic polymers that swells in aqueous media but does not dissolve in it due to the presence of cross-links and exhibits anisotropic expansion [16,17,20,21]. The rationale for using semi-permeable coating over the hydrogel was to prevent the rapid expansion of the expander [22].
Soft tissue expanders can also be classified based on the commercially available forms. (Table 1) [23]. Osmed self-inflating tissue expander (Osmed GmbH, Germany), mentor tissue expanders (Mentor Worldwide LLC, Minneapolis and CUI brand tissue expander (Allergan, California) are some few commercially available tissue expander used for skin and mucosal augmentation.
Table 1: Classification of commercially available soft tissue expanders. View Table 1
The self-inflating hydrogel expanders are available in two shapes:-
1) Cupola: Used especially in small edentulous span (1-2 missing teeth) in the maxilla [23].
2) Cylindrical: Can be used in both maxilla and mandible and is available in 4 different types depending on the volume, length and width [23].
The commercially available self-inflating tissue expanders for intraoral use (Osmed® Germany) are available in the following dimensions:-
Cupola: The expansion volume varies from 0.05 ml with 6 mm diameter (before expansion) to 0.35 ml with 9 mm diameter (after expansion).
Cylindrical: Available in before expansion volume of 0.045 ml, 0.15 ml, 0.25 ml, 0.42 ml and after expansion volume of 0.24 ml, 0.7 ml, 1.3 ml, 2.1 ml respectively.
The ideal soft tissue expander should be made of an inert material causing no toxic reactions to the surrounding tissues. The expansion process should be gradual, slow and as short as possible thereby causing less discomfort to the patient and reducing the risk of tissue perforation. It should result in reduction of treatment time. The ratio of volume after expansion/volume before expansion of the expander has to be as large as possible. An expander should be provided with an option for fixation on the tissues, so that the expander stays in position during expansion. A rounded shape of the expander is preferred. The surface area of the soft tissue expander adjacent to the underlying tissue should be as large as possible. The tissue expander should also be adaptable to form and size of the underlying tissue. The pressure to the underlying structures should be evenly distributed distributed so local peak pressure is minimized. The expander and its components should be easily recognisable in the surgical field during placement and removal. The placement of expander should not be tedious and overtly technique-sensitive [24].
According to Mazzoli R, et al. soft tissue expanders should be easily placed through a small access site and should enlarge over a relatively short time. It should be well tolerated over the long term. There should not be any uncomfortable inflation spikes. The expanders should be resistant to infection, extrusion or inflator complications. Should require minimal intervention, manipulation or revision [25].
Soft tissue expansion involves an inter play of growth factors, cytoskeletal structures and protein kinases (Figure 2). It works via the stretch induced signal transduction pathway.
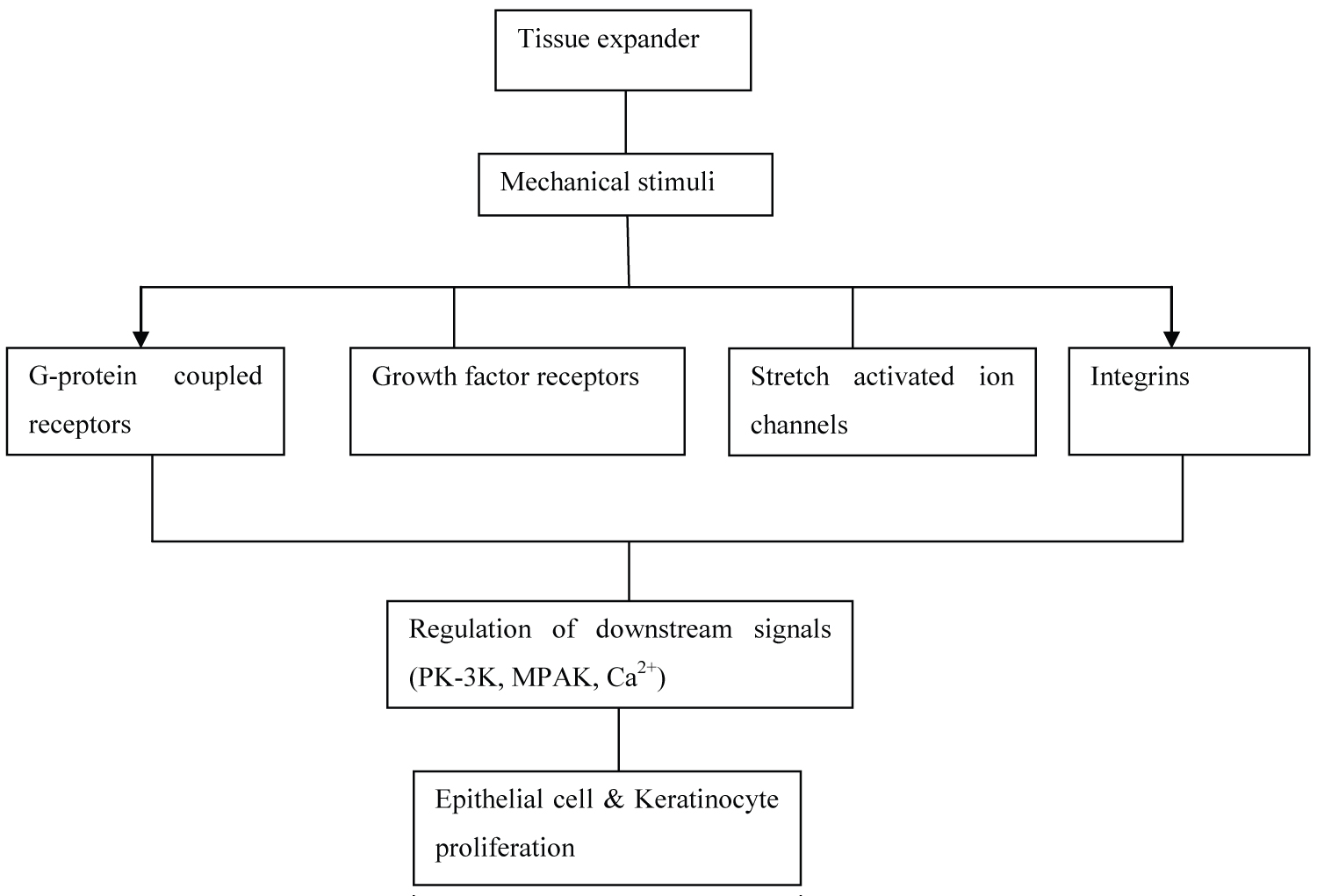 Figure 2: Mechanism of action of the soft tissue expander. The gradual expansion of the expander acts as mechanical stimuli to the overlying tissue resulting in controlled epithelial/keratinocyte proliferation via the inter-play of receptors of the growth factors, G-protein couple receptors, stretch activated ion channels and Integrins.
View Figure 2
Figure 2: Mechanism of action of the soft tissue expander. The gradual expansion of the expander acts as mechanical stimuli to the overlying tissue resulting in controlled epithelial/keratinocyte proliferation via the inter-play of receptors of the growth factors, G-protein couple receptors, stretch activated ion channels and Integrins.
View Figure 2
It was initially proposed that, increase in surface area was due to tissue stretching or "Mechanical Creep". This is seen physiologically in pregnancy, obesity and pathologically in tumor. Stress induced by expansion results in cellular proliferation and DNA synthesis [26].
Growth factors play a very important role in cell homeostasis, so their role in cell proliferation secondary to mechanical strain cannot be ignored. EGF, IGF, TGF help in cell proliferation by governing changes in extracellular matrix and cytoskeletal system [10-12]. EGF is associated with cell proliferative response since a large amount of EGF receptors are found on actin-binding proteins which causes depolarisation of cells leading to influx of cations [26]. TGF-β protein is secreted in response to mechanical stretch stimuli [27]. Stretch induced cell proliferation is matrix dependant, this justifies the role of TGF-β on the amount of type I and IV, fibronectin and other ECM molecules. Mechanical strain also has an autocrine function on the cell by influencing the expression of TGF-β and IL-1 in human keratinocyte that initiates the cascade of cell proliferation [26,28].
Integrins act as mechanochemical transducers that transmit extracellular signals to the cytosol in a force-dependant manner. It results in cell proliferation by activation of varied growth stimulatory proteins such as tyrosine kinase, phospholipase C, protein kinase C.
Cytoskeleton of the cell participates in initiating the mechano-transduction pathway by converting external applied forces into biochemical signals. Rearrangement of actin filaments in the cytoskeleton of cell results in morphological changes of the cell in response to mechanical strain. Actin filament bundles interact with adjacent cytoplasmic membrane via vinculin, talin, paxilin. In mechanical stress, there is interchange of signals through these microfilaments which help in transmitting mechanical signals into the cytosol of cells leading to alteration of extracellular matrix and cell proliferation. This occurs with the activation of integrins that promote formation of focal adhesion complexes which provide a link between integrins-induced receptors and the actin cytoskeleton along with the signal transduction into the cell [26,28].
A number of research studies have emphasized on the role of Ca2+ in stretch induced cell contraction, activation of various intracellular enzymes like the phospholipase C resulting in activation of protein kinase C which results in cell growth. Mechanical strain is known to induce conformational change in cell membrane leading to activation of voltage gated Ca2+ channels that allow the influx of Ca2+ ions and also increase the sensitivity of cells for extracellular Ca2+ ions [26].
Mechanical strain activates phospholipase C thereby activating the phospholipase C-dependant pathway and protein kinase C. The cytoskeleton changes, EGF, Ca2+ are known to activate protein kinase C for cell proliferation [26,27].
Second messenger systems like cAMP are studied extensively by investigators since they are known to play a very important role in cell proliferation probably because there is consumption of cAMP to produce cell protein. It is studied that prostaglandin E2 and cAMP are known to be involved in cell proliferations in response to mechanical stretching [26,27].
These are the various pathways proposed by extensive laboratory studies aiming to understand the response of cells to mechanical stress and stretch. However, still the exact pathway is unknown.
1) Intra-oral tissue expanders are used for soft tissue augmentation before vertical/lateral hard tissue augmentation [16]. Expanders increases the soft tissue volume which helps in the primary wound closure during ridge augmentation procedures and prevents wound dehiscence during healing.
2) Plastic and reconstructive surgeries for congenital defects, anomalies and trauma (e.g. congenital nevi etc) [25].
3) Cleft lip/cleft palate [29].
4) Scars and contractures [30].
Expander size selection: Selection of appropriate size of an expander is obligatory for optimum soft tissue augmentation. The clinician can easily pick an appropriate size of the miniature soft tissue expander with the help of a template provided by the manufacturer. Osmed® Germany provides five types of templates mimicking the five sizes of tissue expanders. Both the ends of the instrument gives a perfect idea to the clinician with regards to the size of the expander before expansion as well as after expansion.
Tunnel preparation: A sub-periosteal or supra-periosteal tunnel is dissected a little more than the size of the expander with the incision a bit away from the expander [16]. The template can be placed in the tunnel to recheck for the correct size of the expander as well as the tunnel dissected.
Fixation of the expander within the tunnel: The expander is slipped into the tunnel and fixated on the bone with a fixation screw. The flat end of the soft tissue expander facilitates screw fixation. Chewing or expanding forces can displace the expanders therefore it is mandatory to fixate the expander. It is necessary to avoid perforations while preparing the tunnel [16]. Placement of expanders in oral mucosa is technique sensitive since the preparation of the tunnel and selection of the appropriate size of the expander needs experience and skill. The tunnel can be closed by two layered wound closure with fine sutures [16]. Placement of the expander within the tissue and mechanism of tissue expansion is depicted in the Figure 3.
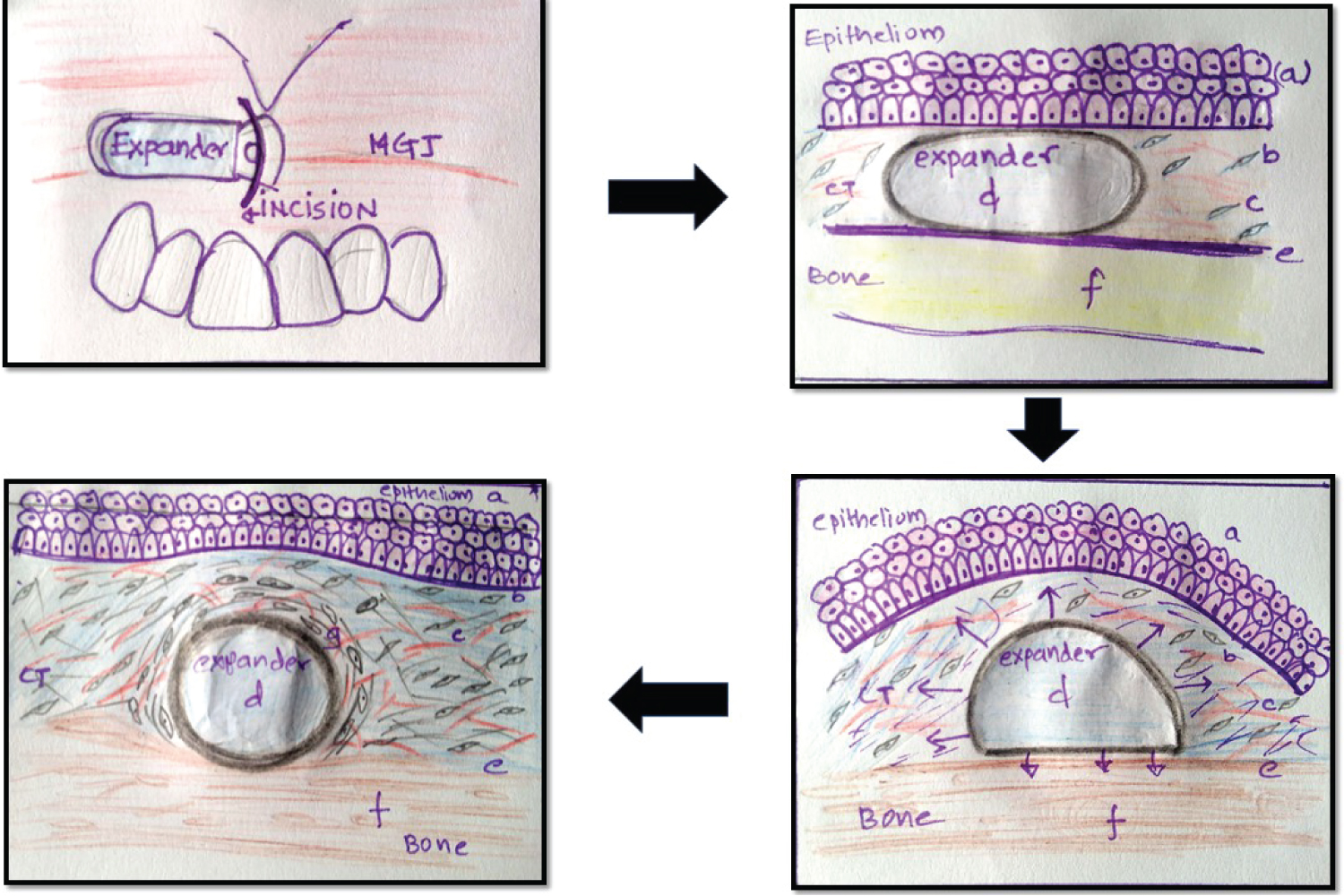 Figure 3: Placement of the expander within the tissue and mechanism of tissue expansion (a- Oral epithelium; b- Fibroblast; c- Blood vessels; d- Intraoral tissue expander; e- Periosteum; f- Bone; g- Fibrous capsule). (A) Small access incision and placement of intraoral tissue expander within the tunnel; (B) Flat (unexpanded) tissue expander within the tunnel; (C) Expansion of the expander within the tissues (convex shape) that stimulates the release of cytokines, changes in the cytoskeleton and protein kinases by mechano-transduction; (D) Increase in the microvessel density and the number fibroblast and collagen within the connective tissue. Placement of tissue expander for a long time can result in formation of fibrous capsule around it.
View Figure 3
Figure 3: Placement of the expander within the tissue and mechanism of tissue expansion (a- Oral epithelium; b- Fibroblast; c- Blood vessels; d- Intraoral tissue expander; e- Periosteum; f- Bone; g- Fibrous capsule). (A) Small access incision and placement of intraoral tissue expander within the tunnel; (B) Flat (unexpanded) tissue expander within the tunnel; (C) Expansion of the expander within the tissues (convex shape) that stimulates the release of cytokines, changes in the cytoskeleton and protein kinases by mechano-transduction; (D) Increase in the microvessel density and the number fibroblast and collagen within the connective tissue. Placement of tissue expander for a long time can result in formation of fibrous capsule around it.
View Figure 3
Removal of the expander: The expander is kept for almost 20-60 days depending upon the location and size of the expander and defect [22,31]. Larger the size of the expander, more is the time it takes to swell [31]. Expanders used for breast reconstruction are kept for around 6 months in the tissues [16].
Clinical studies on the use of intra-oral soft tissue expander for tissue expansion before ridge augmentation: Most of the animal studies on the intra-oral use of soft tissue expander for ridge augmentations have reported that the use of soft tissue expanders results in considerable increase in soft tissue volume that allows efficient ridge augmentation and primary tissue closure with minimal complications [9,32-48]. The soft tissue expanders were left in the animal jaw for minimum of 2 weeks to 6 weeks [9,32-48]. Performing animal studies gave an opportunity to even assess the histologic and histomorphometry features of the soft and hard tissue changes after the use of soft tissue expanders that has proved that the expanders improve the micro-circulation as well as soft tissue volume due to the fibrous capsule formation and little or no inflammatory changes [9,37,42,43,46]. This therefore gave an opportunity for researchers to assess and replicate the results in humans and expand the possibilities of using the expander prior to ridge augmentation. Intra-oral soft tissue expander was not effective in the palatal region which was shown in five studies. It was reported that maximum effectiveness of the soft tissue expander is not appreciated in the palatal region due to the presence of highly keratinised, fibrotic palatal soft tissue and the hard palate beneath. The use of the expander in the palatal region in growing cats have retarded the transversal growth in the anterior part of the bony palate and dentoalveolar structures [32-34,41,42]. Swan M, et al. observed a substantial increase in the palatal soft tissue volume due to the formation of soft tissue capsule with no acute inflammatory response in 6 porcine palate along with attenuation of the palatal bone shelf [42]. The author also advocated that the use of silicon coated intra-oral soft tissue expander allowed controlled soft tissue expansion unlike uncoated expanders that led to animal euthanasia due to mucoperiosteal ulceration by excessive soft tissue expansion [43].
Human studies on the use of intra-oral soft tissue expander and the outcomes are summarised in Table 2 [48-62]. Human studies showed that the advantage in using a tissue expander is that there is considerable soft tissue augmentation both in quality and volume in a short duration of 20-40 days. Byun H, et al. also observed no thinning of the overlying epithelium on account of the expansion of the expander [52]. Mertens, et al. also reported that the expanded tissue was normal in color, texture [56]. The disadvantages of placing the expander sub-periosteally was that a slight amount of bone resorption was observed on account of the expansion process, however this was not observed in the human studies [47]. This procedure is highly technique sensitive especially while fixating the expander and some amount of expansion of the expander takes place. There have been cases of minor perforation of overlying tissue during expansion in-situ [4]. Mild pain, infection, perforation and temporary paraesthesia were some of the complications experienced by patients [48-62]. Placement of soft tissue expander did not result in increase of vestibular depth nor increase in the keratinised tissue width.
Table 2: List of human studies with the use of intra-oral soft tissue expander. View Table 2
Connective tissue capsule formation: A connective tissue capsule is formed around the tissue expander [16,43]. The outer layer of the capsule contains fibroblast and inner layer consist of macrophages [43]. Some researchers believe that the increase in the soft tissue volume is due to the presence of this connective tissue capsule [43]. It has been observed that the outer layer consist of rich collagen fibers, and the innermost layer adjacent to the capsule is richly vascularized after the removal of the soft tissue expander. Abrahamsson removed sub-periosteal expanders 14 days post-insertion without any signs of fibrous tissue encapsulation [54]. Similarly Mertens C also observed a thin capsular around the expander when left for more than 20 days [52]. Soft tissue expanders should not be kept for a long time in situ as the capsule becomes thicker [23]. A subperiosteal placement of soft tissue expander negatively affects the post-expansion augmentation of bone grafts since it is presumed that the periosteum gets converted into fibrous connective tissue that fails to provide viable progenitor cells and also affects the vascularization of the site [23].
Micro-circulation of the soft tissue: The effect of soft tissue expanders on the micro-circulation was assessed by Kaner and Friedman by Laser Doppler Flowmetry [23,43]. It is observed that higher microvessel capacity and blood flow were found in soft tissue in sites where soft tissue expanders were placed compared to sites with no tissue expanders. Von See reported that subperiosteal implantation of expanders resulted in complete ischaemia of periosteum in 14 days leading to conversion of periosteum into a connective which was richly vascularised with a higher density of micro-vessels in the soft tissue surrounding a slowly inflating expander compared to a rapidly inflating expander [56]. Histological investigation reveals that the junction between connective tissue capsule and host tissues shows an increased number of blood vessels, which contributes to an actual increase in the vascularity of the soft tissue and rapid angiogenesis [63].
Effect of soft tissue expander on bone: Expansile forces are transmitted in all directions in the tissue. It was observed that expander left a foot-print on the skull after its removal. Few animal studies demonstrated a decrease in the bone density when the expanders were directly in contact with bone since the underlying bone surface acts as a counter-bearing area [64]. Some studies reported bone resorption or decreased bone density after expander placement [36,52,65,66], while contradictory findings negating this observation was reported in the other studies which showed no bone resorption or even new bone formation [54]. However, Sato, et al. concluded that there was no bone resorption with continuous compressive pressure of < 1.96 kPA [67].
Hydrogel expanders are biocompatible and are considered to be inert with no toxic, mutagenic, or immunologic reactions, not neglecting the fact that the fibrous tissue capsule is formed around the expander due to aseptic inflammatory reactions [20]. An in-vivo study demonstrated that the monocytes neither migrated nor they were tightly attached to the hydrogel, nor did they phagocytose the expander material. The presence of hydrogel expander did not disturb the differentiation processes of monocytes to macrophages or dendritic cells, respectively [20].
The advantage in using soft tissue expanders is that it improves both the soft tissue quality, volume and also increases the vascularity that positively affects the ridge augmentation outcomes. Conversely, expanders come at a higher cost and increased treatment time since it is implanted in the mucosa for 20-60 days before ridge augmentation. It requires two surgical procedures during placement and removal of the expander. The use of soft tissue expanders in the palate is very limited since the palatal tissue is very fibrotic [67,68].
Some complications do occur after use of soft tissue expanders like expander perforation or soft tissue perforation. Subperiosteal implantation causes significant resorption of the underlying bone [50]. Seroma formation was also observed in Ronert study with the use of conventional expanders [22]. Paresthesia of lip is not uncommon due to the subperiosteal tunnel preparation [60]. However such complications can be avoided by higher technical skills and proper selection size of the expander. Candida infections and Aspergillus Niger around and in the soft tissue expander were observed in breast implantation of inflatable expander however its occurrence with the use of osmotic intra-oral tissue expander has not been observed yet [69,70].
A lot of literature is available regarding the use of tissue expanders in animal studies, however expander used in human clinical trials are in countable number. The effect of soft tissue expander on the underlying bone needs to be studied yet, as no significant conclusion can be made. Studies evaluating the effect of soft tissue expander on periodontal clinical parameters assessing the width of keratinized tissue and tissue biotype are required [16]. Further more technical guidelines are necessary regarding the subperiosteal or supra-periosteal tunnel dissection for placement of expander in relationship to the gingival biotype. In vivo study by Varga, to assess the properties of different hydrogel made of acrylamide, acrylic acid or N-isopropylacrylamide found that although N-isopropylacrylamide was one of the most appropriate hydrogel to be used in plastic and reconstructive surgeries, future clinical trials are needed to validate this [71].
An alternative to soft tissue expanders to increase the soft tissue volume before bone augmentation is by using connective tissue graft or other regenerative materials or by making the flap mobile by giving periosteal releasing incision during the bone augmentation procedures.
Improvement in the quality and quantity of the soft tissue that enhances the tension free closure during ridge augmentation can be achieved with the use of soft tissue expanders. More clinical trials authenticating its intraoral use in humans are needed to bring such a promising option forward that will encourage its use in dentistry.
None.
None.
None.