We aimed to compare the 15-day continuous hemodynamic, laboratory and clinical course of patients with ARDS due to COVID-19 who received short-term high-dose and long-term low-dose systemic methylprednisolone.
Two hundred and two patients were recorded to be diagnosed with ARDS due to COVID 19 in the intensive care unit between June 1, 2020 and February 1, 2021. Patients were received systemic methylprednisolone for the short or long term and high or low doses were applied. Age, gender, APACHE II scores, steroid treatment protocol, time for intubation, length of stay (LOS), Neutrophil/Lymphocyte Ratio, C Reactive Protein (CRP), Procalcitonin, Lactate levels and Cytokine filter requirement, the prognosis and total costs were obtained from their records.
It was determined that elderly patients tended to be given low-dose steroids. We observed that high-dose steroid therapy is mostly preferred in extubated patients, while low-dose long-term steroid therapy is preferred in intubated patients (p < 0.05). There was no significant difference between the two treatment protocols in terms of other parameters. It was determined that high-dose steroids affected only CRP levels (p < 0.05).
It was determined that short-term and high-dose methylprednisolone was helpful to decrease CRP values and had no effect on other parameters.
Methylprednisolone, COVID-19, ARDS, Prognosis
Since COVID-19 was first discovered in China in late 2019, the number of people infected continues to rise. If the COVID 19 infection is not eliminated by appropriate and vigorous immune responses, it can result in pulmonary fibrosis, shortness of breath, decrease in O2 saturation, acute respiratory failure syndrome (ARDS), and the death of the patient due to the resulting cytokine storm can happen [1].
Induction of cytokine storm by SARS-CoV-2 has been confirmed in COVID-19 patients in the intensive care unit (ICU), and high plasma levels of inflammatory cytokines have been associated with disease severity and prognosis [2].
Given the increasing incidence and mortality of COVID-19 worldwide, beneficial and effective treatment for patients in the early pulmonary phase is still of great importance. ARDS is the main cause of death in COVID-19 patients and there are no effective specific therapeutic agents for the disease, so glucocorticoids and immunosuppressive therapy can reduce respiratory tract inflammation and prevent cytokine storm and ARDS induction [3].
In this retrospective study, we aimed to compare the 15-day continuous hemodynamic, laboratory and clinical course of COVID 19 patients to whom short-term (3 days) high-dose (10 mg/kg/day) systemic methylprednisolone was administered and to COVID-19 patients to whom low-dose long-term (1 mg/kg/day) systemic methylprednisolone was administered.
This study was initiated as a retrospective cohort study after obtaining approval from Biruni University non-interventional clinical research ethics committee. Ethics committee no: 2021/53-05. ClinicalTrials.gov Identifier: NCT05047874.
Patients over the age of 18 who were hospitalized in the General intensive care unit of Biruni University Medical Faculty Hospital with the diagnosis of COVID 19 between June 1, 2020 and February 1, 2021, and diagnosed with ARDS and over the age of 18 were included in the study. (For the diagnosis of COVID 19; Identification of SARS-CoV-2 by reverse transcription-polymerase chain reaction (RT-PCR) in nasopharyngeal swab or sputum specimens and/or Abnormal lung computed tomography finding (CT), scan finding with < 90% oxygen saturation at rest (bilateral, subpleural, peripheral ground glass opacities) were considered positive.) If the diagnosis of ARDS caused by COVID-19; Patients who received high flow nasal oxygen (HFNO), non-invazive mechanical ventilation (NIV) or invazive mechanical ventilation (IMV) due to acute hypoxemic respiratory failure due to SARS-CoV-2 pneumonia [4] were included. In the context of COVID-19, this definition is fully consistent with the pathophysiological logic that supports the Berlin definition (Table 1).
Table 1: Characteristics of the pragmatic definition of COVID-19 ARDS: "A patient receiving HFNO, NIV, or IMV for acute hypoxemic respiratory failure due to SARS-CoV-2 pneumonia"*. View Table 1
Exclusion criteria from the study were patients with a diagnosis of steroid allergy or those who developed during treatment; pregnant or lactating women; Patients with active malignancies and patients receiving any immunosuppressive agent.
In the study, from the file records of 202 patients hospitalized with the diagnosis of ARDS due to COVID 19 in the General intensive care unit of Biruni University Medical Faculty Hospital between June 1, 2020 and February 1, 2021; patients' age, gender, APACHE II scores, Steroid Treatment Protocol (Those who receive Low Dose Steroid Therapy (1 mg/kg/day, 15 days); Those who received high-dose steroid therapy (10 mg/kg/day, 3 days) were tapered off after 3 days. Continued with low dose for up to 10 days, Intubation Time (Day), Weaning time (days), Hospital stay time (days), Prognosis: (death/survival), Cost (Turkish Lira), and daily Neutrophil Lymphocyte Ratio for 15 days (NLR), CRP (C Reactive Protein), PCT (Procalcitonin), Lactate levels and the need for Cytokine filter (HMP) were recorded.
After obtaining the approval of the ethics committee and the approval of the chief physician, a "data processor" who was not included in the study anonymized the patients who met the inclusion criteria in the study between these dates, and after ensuring that their identity information was hidden, the data in the case registration form were accessed from the computer records and recorded.
The primary aim of the study is to compare the 15-day continuous hemodynamics, laboratory findings and clinical course of COVID 19 patients to whom we administered short-term (3 days) high-dose (10 mg/kg/day) systemic methylprednisolone with those to whom we administered long-term low-dose (1 mg/kg/day) systemic methylprednisolone.
The results were presented for categorical variables as numbers and percentages, for continuous variables as mean ± standard deviation. Comparison of the categorical variables between groups was done using Chi-square or Fisher exact test. For comparison of independent continuous variables between two groups, the Student’s t-test or Mann-Whitney U test was used depending on whether the statistical hypotheses were fulfilled or not. Similarly for dependent continuous variables Paired samples t-test or Wilcoxon signed rank test was used.
SPSS version 21.0 for Windows was used for statistical evaluation (IBM Software, New York, United States), p < 0.05 was considered statistically significant.
The mean age of 202 patients included in the study was 66.64 ± 14.9 years and 57.4% were male. 67.3% of the cases were extubated when they were admitted to the first intensive care unit, and their mean APACHE II score was 26.32 ± 12.1. While the mean day of intubation was 2.19 ± 3.7 days, mean weaning days were 5.86 ± 6.8 days. A total of 78 (38.6%) patients were administered short-term (3 days) high-dose (10 mg/kg/day) systemic methylprednisolone, and it was observed that 51 (25.2%) patients needed HMP. The mortality rate was 59.4%, the duration of hospital stay was 10.99 ± 7.8 days and the cost was mean 30243.378 ± 36596.2 TL (Table 2).
Table 2: Descriptive statistics. View Table 2
The planned 15-day follow-up of 202 patients included in the study was included in the first 10-day follow-up calculations in order not to cause errors in the calculations due to the deficiencies in the continuous data with the increase in deaths after the 10th day. These 10-day NLR, LACTATE, PCT, CRP levels are shown in Figure 1 and Figure 2.
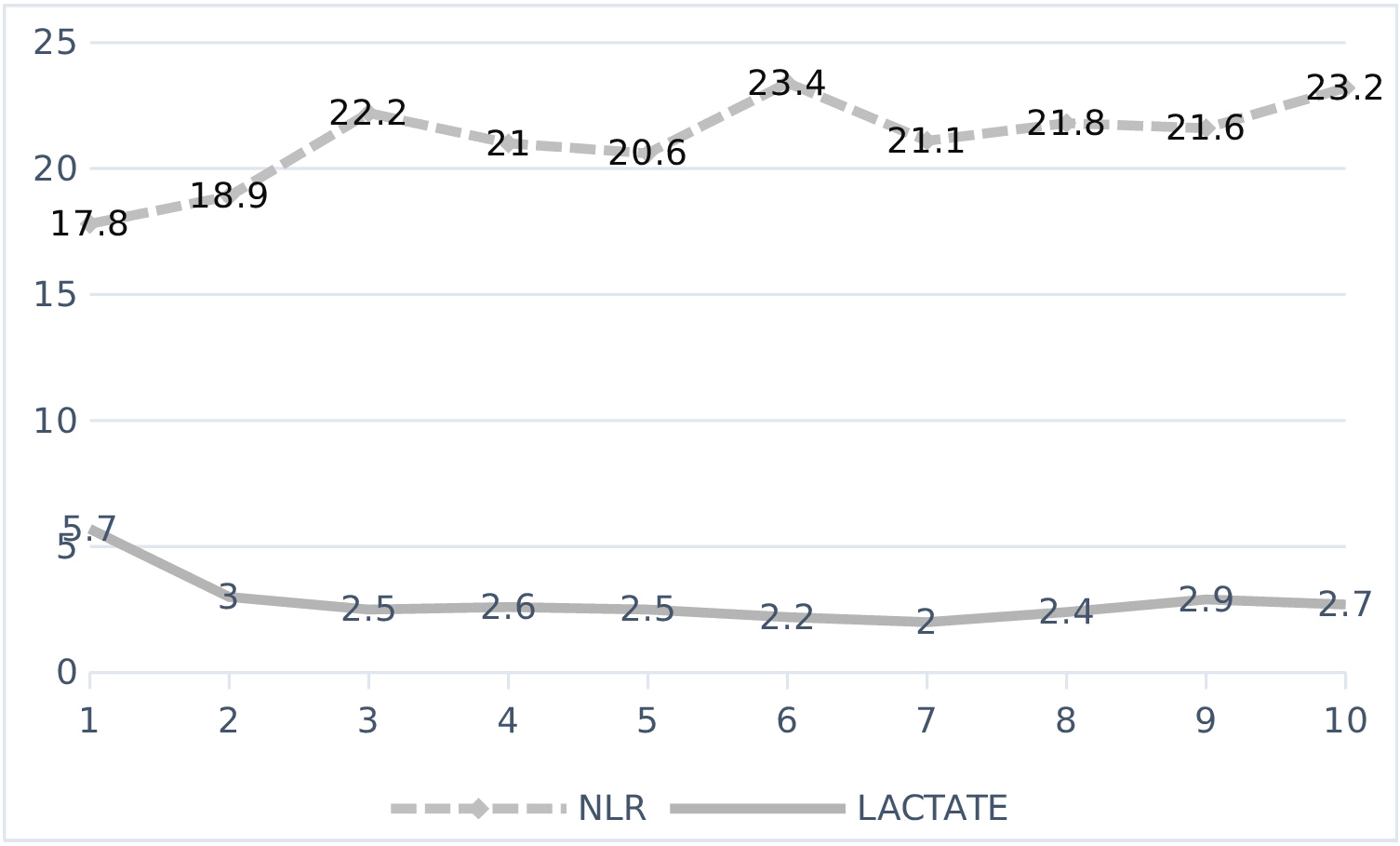 Figure 1: Changes of NLR and LAKTAT over time.
View Figure 1
Figure 1: Changes of NLR and LAKTAT over time.
View Figure 1
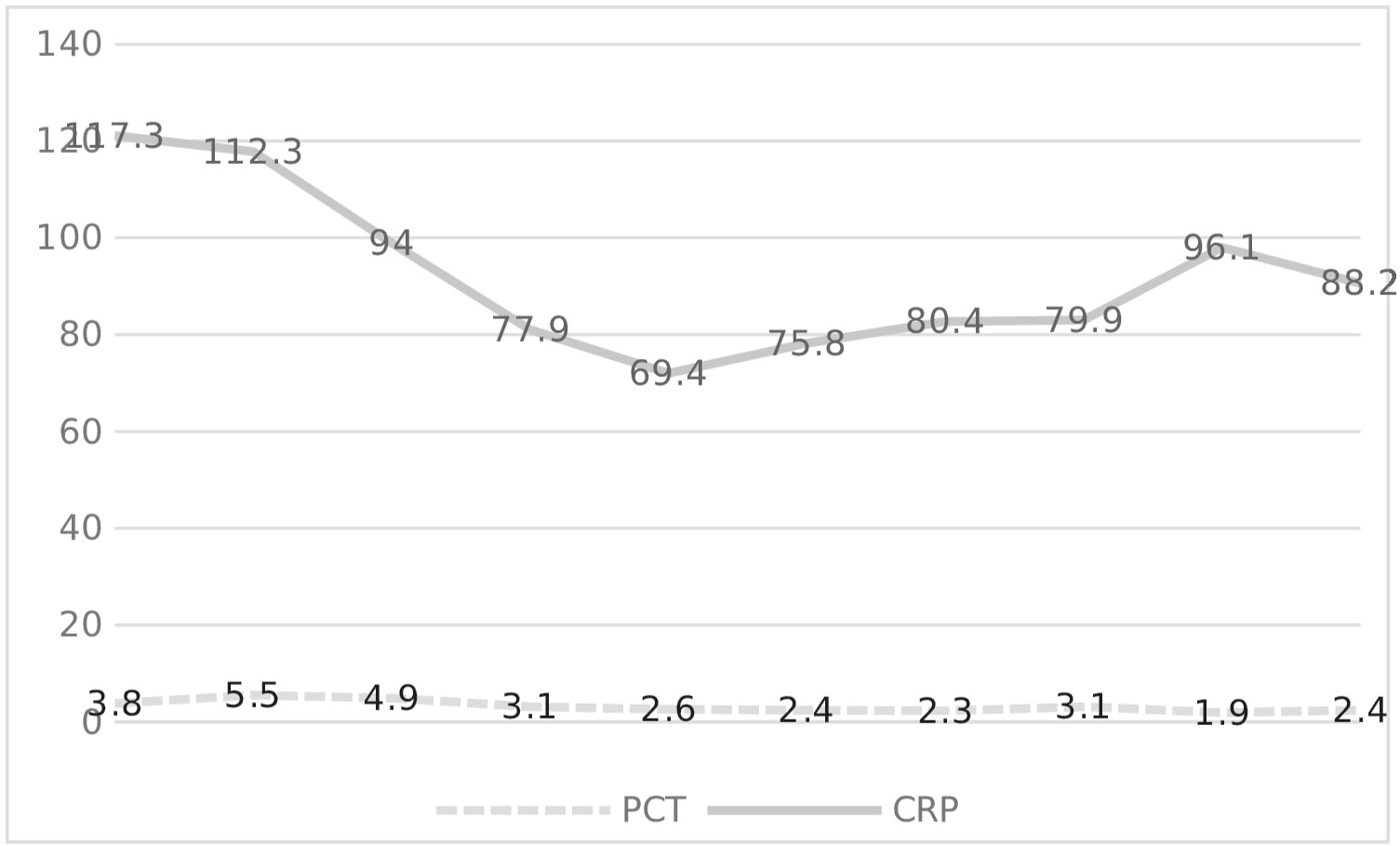 Figure 2: Changes of PCT and CRP over time.
View Figure 2
Figure 2: Changes of PCT and CRP over time.
View Figure 2
The comparison of COVID 19 patients who were administered short-term (3 days) high-dose (10 mg/kg/day) systemic methylprednisolone and low-dose long-term (1 mg/kg/day) systemic methylprednisolone, which is the aim of our study, is given in Table 3. In our study, it was determined that elderly patients tended to be given low-dose steroids. In addition, it was observed that high-dose steroid therapy was preferred mostly in extubated patients, while low-dose long-term steroid therapy was preferred in patients who were intubated (p < 0.05). There was no significant difference between the two treatment protocols in terms of gender, APACHE II, intubation and weaning time, need for hemoperfusion, prognosis, LOS, and cost (Table 3).
Table 3: Comparisons between groups. View Table 3
When we looked at the changes in laboratory values of high-dose and low-dose steroid treatment over time, it was found that high-dose steroid only affected CRP levels (Table 4), but there was no difference in prognosis.
Table 4: CRP levels between groups by time. View Table 4
Methylprednisolone is a glucocorticoid drug used to suppress autoimmune and inflammatory responses in rheumatic diseases [5]. Previously, some studies have been conducted with the thought that the administration of methylprednisolone in the hyperinflammation stage in COVID-19 patients may have possible benefits due to the suppression of the cytokine storm, but the results of these studies are inconsistent. Studies that are claimed to be useful; In a randomized controlled study conducted by Maryam, et al. [2]. They reported that methylprednisolone pulse application at the beginning of the early pulmonary phase of the disease significantly reduced the death rate, improved recovery, and reduced the length of hospital stay [2]. In the same study, they stated that methylprednisolone administration significantly improved pulmonary involvement, oxygen saturation, dyspnea, heart rate, respiratory rate, and inflammatory markers such as CRP and IL-6 in patients, and that methylprednisolone could be an effective therapeutic agent for severe COVID-19 patients hospitalized in the pulmonary phase. Similarly, Li [6] and Sterne [7] concluded in their meta-analysis that systemic glucocorticoids are associated with a reduction in all-cause mortality in critically ill patients with COVID-19. Studies that are claimed to be ineffective; In the studies of HU [8] and Rodrigez Molinerio [1], it was shown that glucocorticoid treatment did not significantly affect the clinical course, side effects or outcome of COVID-19 pneumonia. In a cohort study by Wang, et al. [9] it was shown that patients treated with methylprednisolone had a faster recovery in oxygen saturation, a decrease in CRP and IL-6 levels, and less likely to receive invasive ventilation. However, they did not observe significant differences in mortality between the groups. Hu, et al. [8] mainly focused on the role of low-dose glucocorticoid therapy in COVID-19 pneumonia patients, and in their retrospective study, they showed that glucocorticoid therapy did not significantly affect the clinical course, side effects, or outcome of COVID-19 pneumonia.
In all of these studies, the dose of methylprednisolone was different and 1 mg/kg was used as the high dose. The observed differences may be due to the difference in the amount and duration of treatment, the small sample size, the age of the patients and the severity of the disease. The clinical and laboratory characteristics of the patients and their lung involvement have not been fully determined and have not been reported in these observational studies. In reality, patient characteristics, duration of administration and pulmonary phase are key factors in the effectiveness of corticosteroid therapy. So, et al. [10], who presented the only case report of the use of 1000 or 500 mg/day methylprednisolone for three days in the literature, hypothesized that high-dose corticosteroid treatment could prevent tissue damage and thus reduce the degree of lung damage. 500 mg/day of methylprednisolone, followed by 1 mg/kg once daily, then reduced by 10 or 20 or 30 mg. They used finishing with 10 mg/day oral prednisolone. They stated that starting methylprednisolone intravenously reduced the fever of the patients, 100% survival rate was achieved, and reintubation rates were 0%, followed by complete withdrawal of ventilator support in all cases within seven days. In our study, it was determined that short-term high-dose methylprednisolone only decreased CRP values and had no effect on lactate, PCT, NLR levels, intubation time, weaning time, hemoperfusion requirement, hospital stay, and prognosis.
The limitations of our study are that it is a single-center report, and the number of cases is limited. Since data such as fever, heart rate, inotrope requirement, oxygenation level, which are clinical course findings, are not available in electronic form and can be obtained by handwriting from nurse records, they were not included in the study in order to avoid an erroneous or biased conclusion.
As a result; In this retrospective study investigating the possible benefits of methylprednisolone due to the suppression of cytokine storm in ARDS caused by COVID-19, it was found that short-term high-dose prednol only decreased CRP values, there was no effect on lactate, PCT, NLR levels, intubation time, weaning time, hemoperfusion requirement, hospital stay, and duration of hospital stay and prognosis. For the treatment of COVID-19 ARDS, the timing and dosage of glucocorticoids still require in-depth and systematic research to ensure that they inhibit the inflammatory response in COVID-19 ARDS.
Suna KOC: Conceptualization, Methodology, Visualization, Investigation, Software, Data curation, Writing- Original draft preparation, Writing- Reviewing and Editing; Ilke KUPELI: Software, Data curation, Writing- Original draft preparation, Writing- Reviewing and Editing, supervision.
None.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.