This study reviews patients aged 21 and younger, who presented to the Ochsner Medical Center's Pediatric Emergency Department during the COVID-19 pandemic. A retrospective analysis was completed comparing demographics, symptomology, and laboratory analysis between COVID positive and negative patients.
This study examined pediatric patients presenting to the Pediatric Emergency Department at Ochsner Medical Center between March 11 and May 20, 2020. All children included in the study underwent COVID-19 testing via nasopharyngeal swab. Demographic and clinical information was collected through chart review of electronic medical records, and the Fisher Exact Test was utilized in statistical analysis to determine if clinical characteristics influenced COVID-19 test results in a meaningful way.
In our experiment, 160 patients were included based on symptomatology consistent with COVID-19 infection, and 22 (13.8%) resulted positive. The following variables significantly influenced the COVID-19 test to result positive: Ages 11-18 (p < 0.001), exposure to sick contact (p < 0.001), neurologic symptoms (p = 0.001), and anosmia (p = 0.009). Characteristics which significantly influenced COVID-19 tests to result negative were ages 1-4 (p = 0.040), and White race (p = 0.048). Within the cohort, 6 COVID positive patients necessitated hospital admission, 3 of whom were admitted to the PICU. All 3 PICU admits displayed symptoms of shock, consistent with Multisystem Inflammatory Syndrome in Children (MIS-C).
We found COVID-19 infection rate in children to be 13.8%, which is consistent with national data. We found significant associations between COVID positivity and anosmia, neurologic symptoms, sick contacts and 11-18 year-old age group. COVID negativity was significantly associated with 1-4 year-old age group and White race. While long-term effects from COVID19 are not yet known, our study showed overall flu-like symptoms and no deaths. Yet, Black adolescent patients may be at risk for more severe disease. Continued efforts towards studying transmission and prevention of COVID in children are needed.
COVID, Pediatric, Emergency medicine, MIS-C
SARS-CoV-2, the virus causing the current COVID-19 pandemic, is a novel and rapidly-spreading coronavirus in the same family as SARS-CoV and MERS-CoV (the causes of Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome, respectively) [1]. Corona virus Disease 2019, or COVID-19, was classified as a pandemic on 11 March 2020 by the World Health Organization [2]. As of 17 May 2020, reported COVID-19 cases in Louisiana exceeded 430,100 cases with a death toll of 9,608 [3]. The extent to which New Orleans has been affected marks it as a valuable resource when trying to understand more about COVID-19. Information regarding COVID-19 in children has been limited, conflicting, and lacking in description of the clear symptomatology evident within the adult population [4,5]. This study reviews patients aged 21 years and younger who presented to the Ochsner Medical Center's Pediatric Emergency Department (PED) during the first months of the COVID-19 pandemic. Our retrospective analysis compared demographics, symptomatology, and laboratory analysis between COVID-19-positive and -negative patients.
All pediatric patients who presented to the pediatric emergency department (PED) at Ochsner Medical Center in New Orleans, Louisiana, between March 11 and May 20 of 2020, and subsequently underwent COVID-19 testing were considered for this study. Ochsner Health is Louisiana's largest non-profit, academic healthcare system [6]. The PED has a total patient volume of approximately 15,000 cases per year with an admission rate of 10%.
Demographic and clinical information was collected through manual chart review of electronic medical records (EMR). The health record was used to abstract age, sex, race/ethnicity, insurance, underlying medical conditions, sick contacts, and travel history. The presence or absence of the following symptoms was obtained from each chart: Cough, rhinorrhea, fever, tachypnea, shortness of breath, chest pain, vomiting, diarrhea, abdominal pain, rashes, seizure, and neurological symptoms including headache, presyncope, and weakness. Information regarding vital signs upon PED arrival, diagnostic test results (white blood cell count [WBC], transaminases, C-reactive protein (CRP), procalcitonin and chest radiograph), duration of illness and patient disposition was also collected. Ten percent of all manually extracted records were examined by a second reviewer to ensure accuracy with greater than 90% agreement.
For inclusion in the study, patients were required to have undergone COVID-19 testing via nasopharyngeal swab, and reason for testing was either symptom-based or per hospital admission criteria. Of 242 children tested during our study period, 160 patients (66.1%) met our study's inclusion criteria. Excluded records are composed of duplicate records and asymptomatic children who were tested for admission or transfer requirements. This study obtained IRB approval.
160 nasopharyngeal specimens were tested for SARS-CoV-2 by RT-PCR (n = 16) or RNA amplification (n = 143). The majority of specimens (n = 147) were run at Ochsner Medical Center's microbiology laboratory, while 13 specimens were sent to a national reference laboratory (Mayo, Quest, or University of Washington). All tests have greater than 95% analytic sensitivity and 100% analytic specificity [7,8]. Molecular-based testing for other viral pathogens was incorporated in the study when available, and included influenza A and B, respiratory syncytial virus, parainfluenza virus types 1-4, human metapneumovirus, rhinovirus, adenovirus, enterovirus, B. parapertussis, C. pneumoniae, and M. pneumoniae, as well as corona virus types 229E, HKU1, NL63 and OC43.
Comparing patient's demographic, health status and exposure by COVID-19 classification resulted in the low cell sizes in the cross tabulation, therefore the Fisher Exact Test was used to evaluate these comparisons. For demographic variables with less than 5 in the group, "< 5" is used to protect confidentiality. In comparing symptoms with COVID-19 outcomes the same issue of low cell sizes also necessitated the use of Fisher Exact Test for evaluation of differences in distributions. For comparing the emergency department triage measures, heart rate, respiratory rate peripheral oxygen, and temperature, binary variables were created with abnormal levels coded as "1". Of these Emergency department triage measures, low cell size was only an issue for SPO2 < 92%. For all other triage measures a chi square analyses of the cross tables was used. For all clinical measures an odds ratio with 95% confidence intervals and percentages was also calculated for interpretation of significant results. Alpha was set at 0.05 for significance testing. Data analyzed varied based on available information in patients' charts. Each category's cumulative total was adjusted to account for these differences.
In this cohort, 22 of 160 children (13.8%) tested positive for SARS-CoV-2. Of 160 study patients, 97 (60.6%) were male, and 46 (28.9%, the largest age group) were aged between 1 and 4 years. The second largest age group, 11-18 years-old had one less 45 (28.3%). Of 115 patients for whom race and ethnicity information were available, 64 (55.7%) identified as Black, 33 (28.7%) White, 14 (12.1%) Hispanic, < 5 Asian, and < 5 Native American (Table 1). Of the 15 patients in the COVID-19-positive cohort that also had demographic information, Black patients represented the largest race or ethnic group (73.3%). The majority of the patients (74.6%) were below the 85 percentiles for age adjusted weight. In examining these demographic variables, there was no significant difference in the distribution of positive patients by sex, age adjusted weight percentile or race/ethnic grouping (P > 0.15). However, a closer examination of race found compared to their Caucasian counterparts, patients identifying as Black had a statistically significant higher proportion COVID-19 Positives (3.0% vs. 17.2%, P = 0.05). There was a significant difference in the distribution of positives across age groups (P = 0.004) with the 11-18 age group having the highest percentage of positives with 31.1% of that age group testing positive (Table 1).
Table 1: Association between patient demographics and COVID-19 test results. P-value describes the relationship between patient demographics and positive COVID-19 test results. View Table 1
Significant underlying medical conditions such as asthma, diabetes, congenital heart disease, multiple sclerosis and cancer were seen in 73/160 (45.6%) of patients; the most common underlying condition was asthma in 14/56 (25%). Of those with a significant medical condition there was a smaller percentage with COVID-19 compared to those without a significant medical condition (8.2% vs. 18.4% respectively). This difference was not statistically significant (P = 0.07) (Table 2). Only 14.6% of the group had traveled. Exposures as a result of travel was not a statistically significant influence on testing positive for COVID-19 (P = 0.22). Sick contacts were reported by 47/127 (37.0%) patients. As one would expect, having been exposed to a sick person resulted in higher positive COVID-19 test (P = 0.0002) and an increased risk (OR = 6.37). (See Table 2 of the 15 patients with sick contacts, 11 were residing in the same home as the contact(s), 2 were residing in the same juvenile detention center, 1 reported contact with a COVID-19-positive partner, and 1 resided in the same home as a healthcare worker. Data on sick contacts' test results was not available.
Table 2: Patient characteristics and exposures and their association with positive COVID-19 test results. View Table 2
The most common reported symptom was fever, seen in 96/160 (60.4%) patients, 26 (27.1%) of whom had a maximum recorded temperature greater than 102 °F. Other symptoms that prompted testing were cough, rhinorrhea, tachypnea, hypoxia, shortness of breath, nausea, vomiting, diarrhea, abdominal pain, rash, anosmia, other neurological symptoms, altered mental status, and seizure (Table 3). Frequently cited presenting symptoms in the COVID-19-positive cohort were fever (13/22, 59.1%), headache (9/22, 40.91%), gastrointestinal complaints (9/22, 40.9%), cough (9/22, 40.9%), rhinorrhea/congestion (9/22, 40.9%), and tachypnea (7/22, 31.8%). Anosmia was found to be a significant predictor of a positive COVID-19 test result (p = 0.009) with positive COVID-19 reported by 75% of patients with anosmia. Other neurological symptoms were reported by 12 patients. Of COVID-19 positive, 31.6% of patients exhibited other neurological symptoms compared to 8.3% who did not (P = 0.0008). Similarly, headache presented more frequently in COVID-positive patients than COVID-negative (40.9% vs. 9.4%, P = 0.0004).
Table 3: Symptoms and their association with positive COVID-19 test results. View Table 3
Emergency department vitals were also included in this study. These included heart rate, respiratory rate, peripheral oxygen level and temperature. None of the ED vitals were predictive for COVID-19 in these children (P > 0.44) (Table 4).
Table 4: Emergency Department Triage vitals and their association with positive COVID-19 test results. View Table 4
Diagnostic tests were available for 10/22 (45.5%) COVID-19-positive patients (Table 5). CRP elevation greater than 10 mg/L was found in 4/22 (18.2%) patients, with 3 of these significantly elevated (> 50 mg/L). Aspartate aminotransferase (AST) levels greater than 50 U/L was found in 2 (9.1%) patients. 14 patients (63.6%) underwent chest radiography; the majority of these studies (10/14, 71.4%) were unremarkable. Acute findings included opacifications, cardiomegaly, and atelectasis (Table 3).
Table 5: Laboratory Results in COVID-19-Positive Patients (n = 10)*. View Table 5
Within the positive cohort, 27% (6/22) of children required hospital admission. Three children (50%) were admitted to the pediatric intensive care unit (PICU) for cardiovascular and respiratory support. The remaining children were admitted to acute care beds due to concerns for sepsis, respiratory distress, or for mental status observation.
All patients admitted to the PICU were between the age 12 and 17. All were diagnosed with Multisystem Inflammatory Syndrome in Children (MIS-C), with symptoms consistent with acute kidney injury and Kawasaki-like illness. Myocarditis with decreased cardiac function was evident in 2 of the 3 patients; both eventually required inotropic and respiratory support. All three children received IVIG. Two of the three had previously been in excellent health; the third had a history of hyperthyroidism treated with methimazole. All three children were discharged home after 10-14 days of hospitalization with return to pre-illness health on follow up.
The novel SARS-CoV-2 virus is an emerging pathogen with severe health implications, including death, in affected individuals [1,9-11]. Since its emergence in the United States in January 2020, there have been over 5.8 million cases with over 178,000 reported deaths [12]. The Louisiana Department of Health reported its first case of COVID-19 on March 9, 2020, and by March 26 New Orleans Parish had the highest COVID-19 death rate of any US county [13]. New Orleans' death rate was approximately twice that of Richmond County, New York, the county with the second-highest death rate, and higher than that of Manhattan, despite being approximately a quarter of the population size [13]. As of August 26, 2020, Louisiana remains a hotbed of infection during the COVID-19 pandemic and provides vital information regarding its ongoing spread [13]. Having a developed understanding of COVID-19 in children is particularly important, as children may play a significant role in community transmission, but often present with less severe clinical symptoms than their adult counterparts, resulting in an appearance of minor or no illness. Participation of these well-appearing children in shared activities may pose a high risk for widespread virus transmission [1].
Our descriptive study highlights clinical and demographic characteristics of children tested for SARS-CoV-2 during their presentation to the PED at Ochsner Medical Center in New Orleans, Louisiana, during the early phases of the COVID-19 pandemic. Approximately 14% of children in our cohort, who presented with symptoms consistent with corona virus infection, had COVID-19 isolated from their nasopharynx. This incidence of infection is consistent with that from other studies, with percentages ranging from 10% to 20% [1,14,15].
According to the US census, Louisiana has one of the largest Black populations in the United States, at 32.8% [16]. This is particularly important as adult studies indicate that Black individuals are at higher risk for severe infection from the novel SARS-CoV-2 virus, and in Louisiana the percentage of COVID-related deaths occurring within Black communities has ranged from over 70% to 38.7% [3,17,18]. Even as one of the largest Black communities in the US, those who identify as Black comprise less than a third of the population in Louisiana, yet accounted for the most frequently presenting race at 73.3%. When compared to White patients in this study, Black patients were significantly more likely to test positive for COVID-19 (3.0% vs. 17.2%, P = 0.05). Conversely, White race was found to be significantly associated with absence of COVID-19 infection. More than half of our study's hospitalized children with COVID-19 were Black, and three (50%) of these children were diagnosed with MIS-C. Two of three children diagnosed with MIS-C required PICU admission for cardiovascular support. This is comparable with other recently published studies. Bhumbra, et al., observational study showed a similar disproportion of Black children, 74% of who required hospital admission [19]. All intensive care admissions in a study by Bandi, et al. were Black children [20]. A Lancet study showed that 6/8 children admitted for MIS-C were of Afro-Caribbean descent [21]. An article published in JAMA Network Open by Lee, et al. further highlights this disparity in black children, who only constitute 22.2% of the NYC population and 19.9% of COVID-19 hospitalizations among patients younger than 20 years, represented 34.4% of patients with MIS-C that were admitted [22]. In the MMWR published by CDC in August 2020, this racial difference was again confirmed, but also noted that forty-two percent of children in their analysis had one or more underlying medical conditions, with higher prevalence among Hispanic and black children, listing obesity as the most common [23]. Further studies are needed expand on underlying medical or social cause of said disparities leading to increased hospitalization and more significant illness Figure 1.
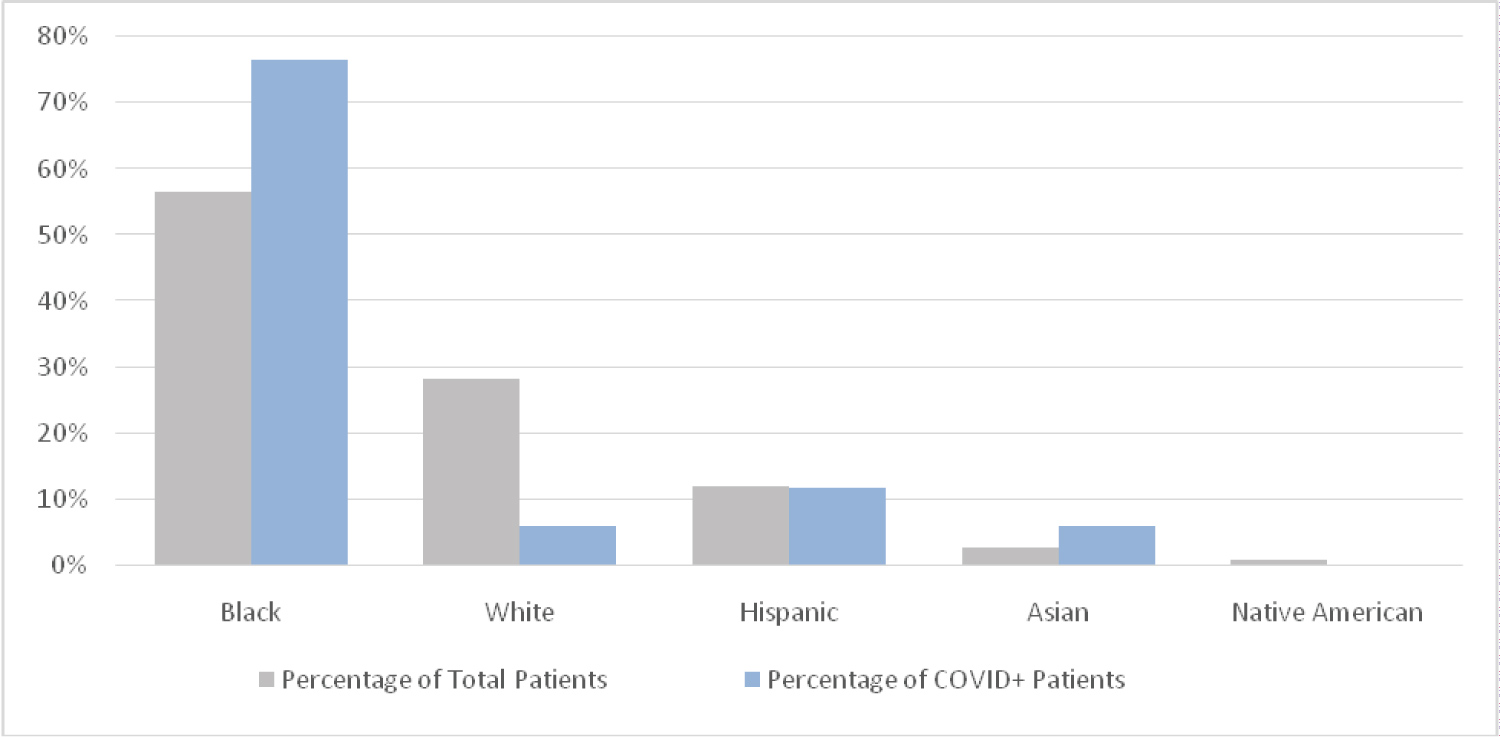 Figure 1: Patient distribution by race/Ethnicity. View Figure 1
Figure 1: Patient distribution by race/Ethnicity. View Figure 1
As shown in Figure 2, the majority of our positive infection cohort includes adolescents (ages: 11-18) and children under 4 years of age, portraying a similar distribution to a study from Riley Children's Hospital in Indianapolis, published in Pediatric Critical Care Medicine [19]. Other studies have also noted higher infection rates in adolescents and increased illness severity in this cohort [19,24,25]. The correlation between age and disease severity raises the question if adolescents with COVID-19 disease experience illness progression similar to adults [24].
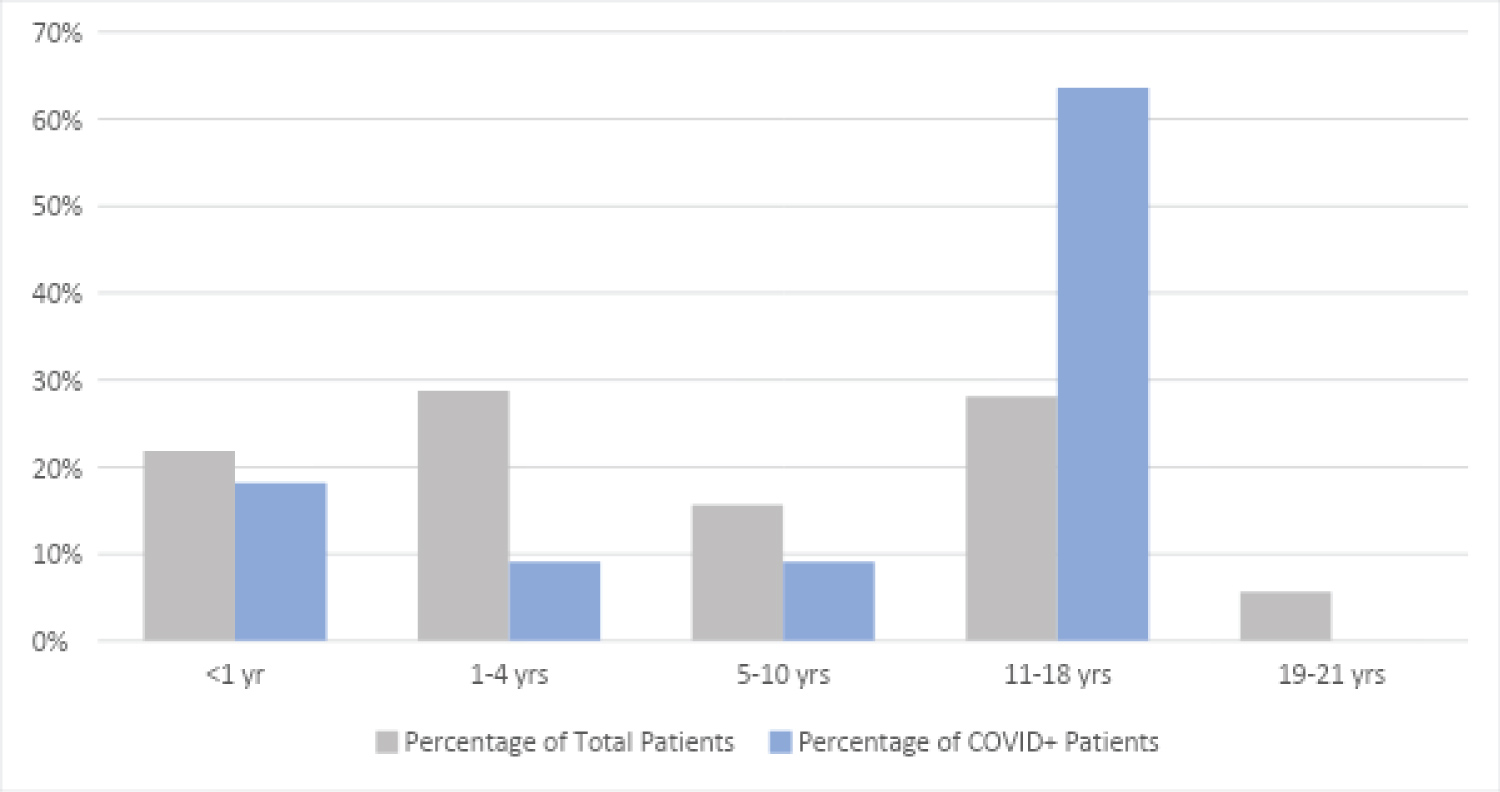 Figure 2: Patient age distribution. View Figure 2
Figure 2: Patient age distribution. View Figure 2
The most common presenting symptom in our study was fever, which was present in almost 60% of the patients tested. This is similar to a recently published CDC study, which noted 56% patients presenting with fever [26]. Cough was reported in just less than half of our patients; cough and fever are the two most commonly reported symptoms in similar studies [27]. We found anosmia to be a statistically significant predictor of positive COVID-19 test results (p = 0.009). Although anosmia was reported as a symptom by less than 14% of the positive cohort, it was never cited as a symptom in the negative cohort and may provide a high degree of specificity when ruling out other diseases. A recently published case series also reports anosmia in pediatric patients as a specific indicator of infection [28].
Headache was significantly associated with COVID-19 positivity (p = 0.0004). In the positive result cohort, 40.91% presented with headache, which is significantly higher than reported in previous studies [29-32]. Literature on adults and children has reported headaches as being one of the most frequent neurological symptoms associated with COVID-19 [29,30]. Other studies describe subsets of patients with COVID-19-associated neurological disease ranging from stroke, seizures, hypoxic encephalopathy, and Guillain-Barre Syndrome [33]. It is theorized that this process occurs secondary to other organ dysfunction, electrolyte derangement, and/or medication side effects, and only rarely from direct infiltration of the virus into the central nervous system [33]. While the majority of children with COVID-19 have recovered without apparent sequelae, the potential for long-term complications is not yet known [1,10].
When reviewing diagnostic data, while not statistically significant in our study, elevated CRP was noted in several patients in the positive cohort and has been noted in other studies to be predictive of more severe disease [24]. Interestingly, of the 4 patients who had elevated CRP in our positive cohort, 3 of these patients required PICU admission. In addition, the majority of our positive patients had unremarkable chest imaging, similar to a study published in Pediatric Pulmonology [34]. A systematic review previously mentioned noted in their findings that 73.9% of patients had chest computed tomography performed, of which only 32% were positive [35]. Although we did not find this statistically significant, our findings support the current trend of unremarkable chest imaging in our pediatric COVID-19-positive patients [24,34,35].
Our study found that a significant number of our COVID-positive cohort reported having been in contact with another sick individual. This ranged from a close family member such as a parent or sibling, to a healthcare worker at a child's birthday party. A systematic review published in April 2020 similarly noted that most children who are infected have a positive family contact [27]. In an article published in Pediatrics in January 2021, further evidence discussed regarding that referenced household contact tracing studies arguably provide the best evidence regarding pediatric susceptibility to SARS-CoV-2, in which the intensity of exposure between household contacts is higher and more consistent than outside the home [36]. Seventy-two percent of COVID-positive patients recalled being in proximity to a sick contact, which significantly influenced the COVID-19 test result (p < 0.001). In addition to known respiratory droplet transmission, there is evidence of fecal shedding of COVID-19 for up to 4 weeks following infection raising concerns about younger children and infants as vectors and resulting in significant implications regarding the reopening of daycares, schools, and other public places [27,37-39].
Although small sample size was a limitation of this study, it is not inconsistent with the relatively low number of children who seem to contract COVID-19 or at least display symptoms promoting an emergency department visit. Laboratory value availability was also limited for many patients due to the higher index of suspicion generally required to warrant laboratory investigations or radiation exposure in pediatric patients.
Our study provides data, including test result rates, symptomatology and race distribution, that are both consistent with and add to the limited information currently available regarding pediatric COVID-19 infection. We found headache, anosmia, and sick contacts to be significantly associated with testing positive for COVID-19. The Black community has been, and continues to be, disproportionately affected by COVID-19, at times comprised more than 75% of COVID-positive tests yet accounting for less than one third of the Louisiana population. When compared to White patients, Black race did increase the likelihood of testing positive for COVID-19. Racial disparities are not unique to COVID-19 and should be addressed vigilantly. Continued investigation into transmission, prevention and treatment of COVID-19 in children is needed.