A 61-year-old man with amyotrophic lateral sclerosis (ALS) on tracheostomy positive pressure ventilation was brought to the emergency room due to decreased consciousness. He was diagnosed with hyperosmolar hyperglycemic nonketotic coma (HHNC) with severe systemic hypotension. He exhibited an adequate response to treatment of continuous intravenous insulin infusion and fluid replacement. On the third day, he developed acute limb ischemia due to extensive occlusion from the left common iliac artery to the femoral artery. He underwent emergency thrombectomy to rescue the limbs. Although glucose intolerance and dyslipidemia are recognized as possible complications in ALS, HHNCs that develop in ALS are very rare in clinical settings. Acute occlusion of the lower limb artery is still very critical and deteriorates not only the limbs, but also increases the risk of mortality. Careful attention should be paid to complications of arterial thromboembolic events during the first few days of treatment of HHNC in patients with ALS.
Acute lower limb ischemia, Hyperglycemic hyperosmolar non-ketotic coma, Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a progressive disease that affects the motor neurons and results in the development of non-neuronal comorbidities in addition to neuromuscular symptoms [1,2]. Glucose intolerance and dyslipidemia are indicative of metabolic abnormalities in patients with ALS. However, hyperosmolar hyperglycemic state (HHS), which is a serious hyperglycemic emergency, is a rare complication of ALS [2].
Here, we report a rare and critical case of ALS with hyperosmolar hyperglycemic nonketotic coma (HHNC) which was complicated by acute occlusion of the lower limb artery during the treatment of HHNC.
A 61-year-old man was diagnosed with amyotrophic lateral sclerosis (ALS) 21 years prior. He developed quadriplegia and respiratory muscle paralysis and introduced tracheostomy positive pressure ventilation five years ago. Although an increase in blood glucose level was recently observed, he was often hydrated with soft drinks in addition to tube feeding during home care. In order to reduce consciousness during home-visit nursing care, the patient was moved by ambulance. His blood pressure was 55/31 mmHg, and his heart rate was 137 bpm. His level of consciousness was calculated using the Glasgow coma scale (GCS), giving the score of E4VTM1. Laboratory data showed hyperglycemia (blood sugar, 1159 mg/dL) without acidosis (Table 1). The calculated plasma osmolality was 397 mOsm/kgH2O. His level of consciousness later deteriorated to a GCS score of E1VTM1. He was transferred to the ICU with a diagnosis of HHNC and began continuous intravenous insulin infusion, fluid replacement (Figure 1A), and was administered noradrenaline. An escalating noradrenaline dose was required with a maximum dose of 0.2 micrograms/kg/min. Hypovolemic shock and hyperlactacidemia improved after aggressive intravenous fluid therapy. The dose of noradrenaline was smoothly reduced and stopped within 10 h. Blood sugar levels were successfully decreased to 200 levels within 12 h from the start of the initial treatment (Figure1B). Positive fluid balance was calculated as 4647 mL on ICU day1 and 505 mL on ICU day 2. On the third day, his left lower limb showed a poor color tone, cold sensation, and blistering, with no complaints of other symptoms. Contrast-enhanced computed tomography (CT) showed extensive occlusion from the left common iliac artery to the femoral artery (Figure 2), and a diagnosis of acute lower limb artery occlusion was made. Cardiovascular surgery received urgent consultation for the management of severe limb ischemia. He underwent emergency thrombectomy on the same day. The skin color and blistering of the affected limb were corrupted within a few days (maximum creatinine phosphatase kinase level: 434 IU/L). The limb skin condition later improved without ulceration or gangrene. Thus, his limb was rescued from amputation, and he returned home 7 weeks after operation with antiplatelet drugs.
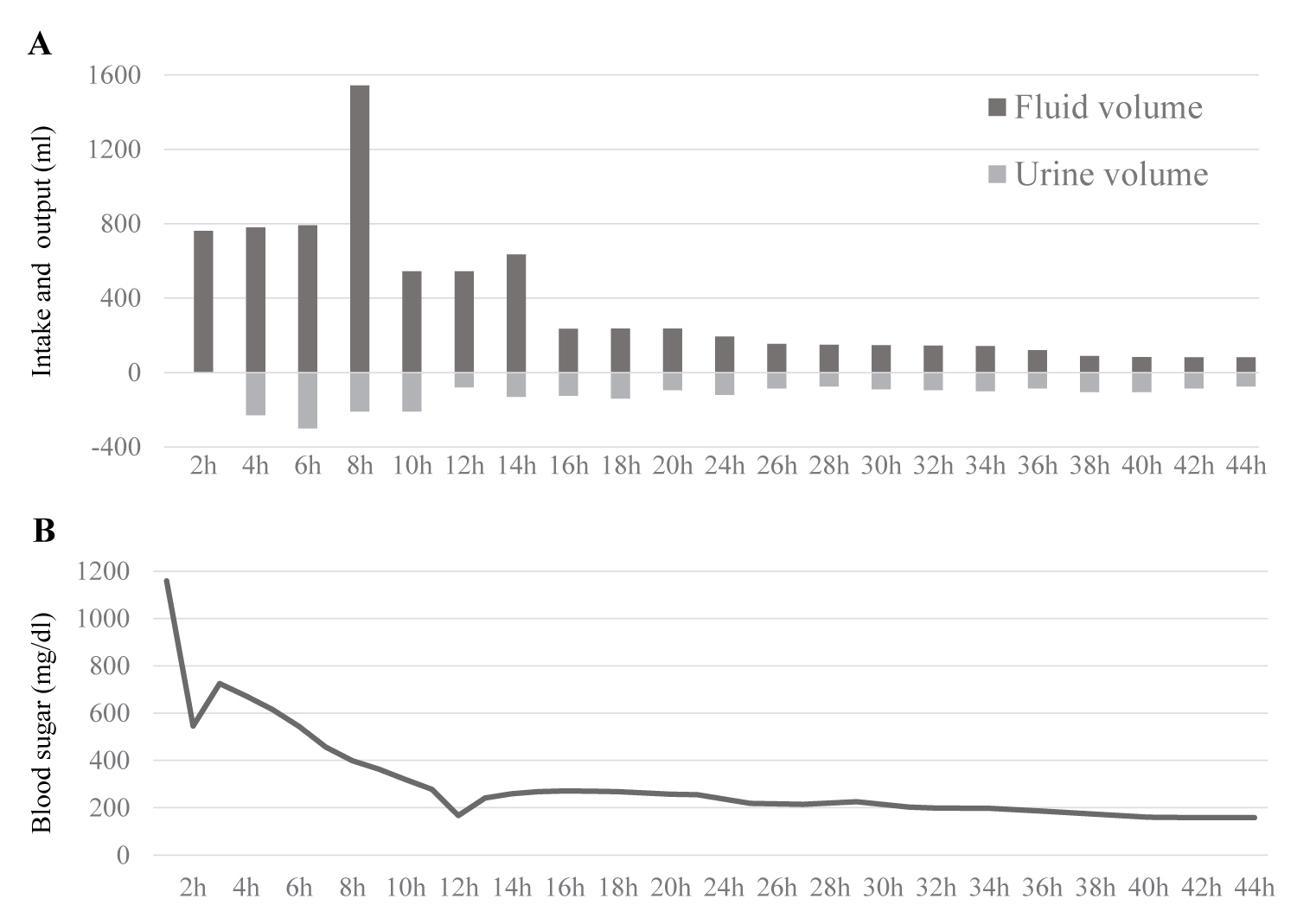 Figure 1: Time course of blood sugar and water balance with changes in fluid volume and urine volume after admission.
View Figure 1
Figure 1: Time course of blood sugar and water balance with changes in fluid volume and urine volume after admission.
View Figure 1
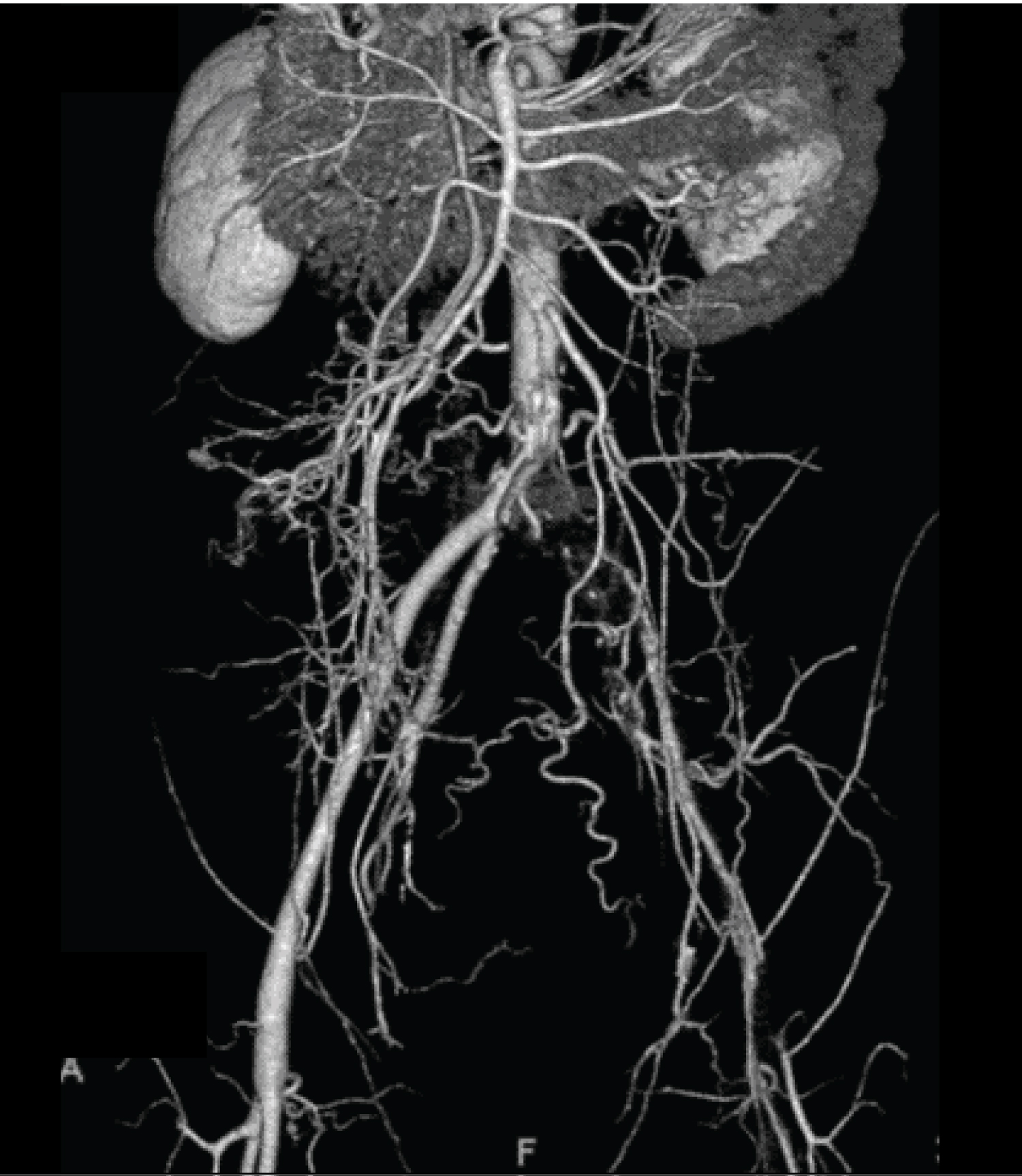 Figure 2: CT angiogram shows occlusion of the left iliac artery.
View Figure 2
Figure 2: CT angiogram shows occlusion of the left iliac artery.
View Figure 2
Table 1: Laboratory data on admission. View Table 1
HHS is characterized by severe hyperglycemia, hyperosmolality, and dehydration, in the absence of significant ketoacidosis [3]. About 1% of emergency department (ED) patients with diabetes related conditions have HHS [4-6]. Extreme dehydration and metabolic disturbances develop over many days in patients with HHS than that in patients with diabetic ketoacidosis (DKA) [7]. As the condition progresses, neurological symptoms such as somnolence and coma appear. HHS has been reported to have a high mortality rate, ranging between 5%-40% [3,7].
Evidence has accumulated on metabolic abnormalities at various stages of ALS [8]. Glucose intolerance and dyslipidemia are frequently reported in patients with ALS [1,2]. In the initial stages, abnormal hypermetabolism specific to ALS is caused by rapidly progressive weight loss [8,9]. During the advanced and chronic stages of ALS with ventilatory support, the calorie balance turns to hypometabolism [8,10], resulting in severely decreased physical deconditioning.
In patients with advanced ALS, severe muscle wasting increases insulin resistance, as the skeletal muscle is the largest glucose consumer in the body [8].
Shimizu et al. demonstrated an abnormally low insulinogenic index in several ALS patients and suggested that the abnormally decreased early phase insulin secretion might have been compensated for by low calorie consumption, and have thus facilitated hyperglycemia during HHS [8].
A recent study by Araki, et al. elucidated that the dysfunction of TDP-43, which was discovered as a pathological molecule of ALS, is involved not only in the degeneration of motor neurons, but also in the decrease in insulin secretion [1]. According to previous studies, TDP-43 is a novel regulator of insulin secretion, and it has been proven that the loss of nuclear TDP-43 is the underlying cause of impaired early phase insulin secretion in patients with early stage ALS [1].
HHS is considered to be a possible complication in such patients; however, HHS in patients with ALS is rare in clinical settings. In our literature search, only one description of five cases of HHS in ALS patients [8] was found. Since HHS is a rare but life-threatening cause of diabetic patient admission to the ICU [11], diagnosis must be made promptly, treatment intensively monitored, and a specialist diabetes team involved as soon as possible after admission [12]. In the absence of an insidious presentation of the disorder, clinicians are frequently misled into dangerously inadequate therapeutic interventions. Patients with ALS need to be systemically managed with the recognition that they have a risk of worsening diabetes and the most serious acute metabolic complication of HHS.
HHS has been recognized as a risk factor for venous thrombosis. The risk of arterial thrombosis in HHS is briefly described in the British guidelines for HHS [7]. The 2014 Oxford Handbook of Clinical Medicine advises the use of thromboprophylaxis for DVT in cases of hyperosmolar nonketotic diabetic coma [13-15]. Although arterial and venous thromboses have long been considered to be distinct entities, with different pathogeneses [16], it is recommended that prophylactic treatment for vascular events with low-molecular-weight heparin be administered, without distinction between venous and arterial thrombosis [7].
A pilot study indicated that HHS is in a pre-DIC state during coma, and intra-arterial thrombus is likely to occur for several hours to several days [17]. Gresele, et al. proved that short-term hyperglycemia induces a significant rise in plasma von Willebrand factor (vWF), whereas after 4 h of euglycemia, no changes in vWF were observed [18]. High shear stress-induced platelet activation is strictly dependent on plasma vWF [19-21]. Thus, acute, short-term hyperglycemia induces an increased activation of platelets that are exposed to high shear stress with a mechanism dependent on a raised plasma level of vWF [18]. They concluded that this may potentially contribute to precipitating arterial thrombotic occlusion at the stenotic site [18].
The incidence of acute lower limb ischemia in diabetic patients with severe dehydration and hyperosmolar states has been poorly reported [4,11,22]. The mortality rate of acute arterial occlusion is reported to be 10%-28% [23]. If prompt and appropriate treatment has not been provided, acute occlusion of the lower limb artery is critical, and could deteriorate not only the limbs, but also the prognosis of life.
Megarbanea, et al. analyzed 17 patients with HHS in the ICU. The incidence of acute lower limb ischemia during HHS treatment was 24% (4/17) [11]. The factors associated with the onset of this complication were severe dehydration, catecholamine-treated shock, and identification of cholesterol embolism [11]. According to previous case reports, acute lower limb ischemia with arterial thrombosis events occurred on the first, second, and third days after admission [4,22]. The latter case was a very rare case of multiple arterial thrombosis, including brain infarction. In each case, limb salvage was possible [22]. These entities may suggest the possibility of preventing arterial thrombosis in various organs and extremities by rapid therapy for euglycemia. Attention should be paid to the complications of arterial embolisms during HHS treatment.
In this report, the patient received full systemic anticoagulation with low molecular weight heparin for only 2 days after surgical intervention for arterial thrombosis of the lower limb. Simultaneously, antiplatelet agents were administered via an enteral route or by injection during admission. Fortunately, the patient recovered his limb and did not experience additional arterial thrombosis.
This is the first case report of acute occlusion of the lower limb artery in a patient with ALS during treatment for HHNC. Rapid recovery from hyperglycemia and severe dehydration may help in withdrawal from the pre-DIC state with hyper-reactivity of platelets and hypercoagulation. Further research will be necessary for the establishment of prophylactic treatment for arterial thrombosis in HHS.
HHS is considered a possible complication in advanced ALS. When HHS is present during a pre-DIC state during coma, intra-arterial thrombus is likely to occur for several hours to several days. Careful attention should be paid to the complications of arterial occlusion during the treatment of HHNC in patients with ALS.
The authors declare that there is no conflict of interest regarding the publication of this paper.