Several advancements are made in the field of immunotherapy for enabling an effective cure for cancer. Treatments are being approached with multipronged approach with combination therapies as well as by gene therapy. Cancer incidence however progressing further mainly due to the changes in lifestyle. Epidemiological surveys indicate that cancer incidence is being more predominant in females; in particularly breast cancer is the most common diseases throughout world. However, the treatments attempted to deal with breast cancers especially triple negative cancer hasn't shown considerable success. NK cell therapy has shown some progress in initial stages of breast cancer, not in metastatic breast cancer. This is due to inefficient NK cells anti-tumor response. The anti-tumor response of NK cell is varied as based on exposed to a range of cytokines and/or mitogen.
We have evaluated the anti-tumor immune response of NK cell by using IL2 combination with less virulent Streptococcus pyogenes of human origin (OK432, Picibanil, manufactured in Japan). Cell doubling time, Immunophenotyping, mRNA expression and cytotoxicity of NK cells against MDA-MB-231 breast cancer cell line were performed and compared between with and without OK432.
Our results show that there was no difference in terms of NK cell proliferation between with and without OK432 expansion and an average cell fold was increased to mean of 200 ± 3.5. However, the surface marker of CD56+ and CD16+ were remarkably expressed on NK cells cultured with OK432 (85.5 ± 0.9, 60 ± 0.7) than the cells cultured without OK432 (65.5 ± 0.9, 50 ± 0.7). Subsequently, the gene expression pattern of Perforin and NKG2D was significantly increased in cells cultured with OK432 as compared to without OK432. Cytotoxicity analysis revealed that NK cells cultured with OK432 lysed the target cancer cells faster than the cells cultured without OK432 in time and dose (E:T ratio) dependent manner.
Overall our results suggested that OK432 has a vital role to play in NK cell augmentation for effective cell-mediated lysis.
NK cell, Breast cancer, OK432, Cytotoxicity, MDA-MB-231
Breast cancer is the most common invasive cancer in females; it remains the most widespread type of cancer and statistically the second leading cause of cancer related death [1]. Numerous of studies are underway for identifying therapeutic strategies to combat cancer using chemotherapy and radiotherapy [2,3]. Though, the treatment of triple negative breast cancer (TNBC) such as MDA-MB-231 is very challenging due to its molecular heterogeneity [4], several targeted therapies such as tamoxifen, trastuzumab and anastrozole have proven to be effective for breast cancer [5,6] but the treatment for TNBC is limited [7]. As a result, development of treatment against the aggressive breast cancer subtypes is challenging [8].
Recently, ranges of rehabilitation targeting breast cancer have been developed, particularly in the field of cancer immunotherapy which exploits the influence of the immune system to treat cancer. The use of CD56+ or CD16+ natural killer (NK) cells therapy has acquired wide interest to identify and kill target cancer cells without prior sensitization, acts an important player in immunosurveillance and cancer defense in vivo [9-11]. Hence, NK cell-based immunotherapy may be a promising therapeutic strategy against cancer [9]. There have been several studies investigating the role of NK cells on solid tumor [12,13], however, the ability of NK cells to recognize and kill tumor cells is significantly impaired in cancer patients [14-16]. NK cell has received considerable attention to increase the activity of immunity by a number of agents such as the virus, tumor cells, BCG and IFN [17-20].
In addition, studies reported that the biological agent "OK432" (Picibanil, manufactured in Japan) which derived from a group A Streptococcus pyogenes of human originis widely used for cancer treatment and has shown better efficacy in malignant patients [21,22]. It is detected that OK432 augment the cytotoxic activity of human natural killer (NK) cells by induction and activity of natural killer cytotoxic factors (NKCF) for NK target specificity for lysis [23]. Based on the evidence and with keen research interest on the OK432 activity, the present study was designed to evaluate the anti-tumor immune response of OK432 on NK cell against TNBC cell line (MDA-MD-231).
Peripheral Blood (PB) was collected from three (3) healthy donors after informed consent was obtained in accordance with local regulatory guidelines. Approximately 30 ml of PB was collected in heparin tubes by venipuncture and transported to the lab within 6 h at room temperature. All samples were subjected to microbial screening and approximately, one (1) mL of the blood was incubated throughout the culture period in bacteriological incubator to detect contaminations if any. Peripheral blood mononuclear cell (PBMCs) was isolated by lymphoprep (AXIS-SHIELD PoC AS, Oslo, Norway) density gradient medium and centrifugation at 2000 rpm for 20 mins. Isolated PBMCs were washed twice with phosphate buffered saline (PBS) and the cell pellet was counted.
The isolated PBMCs were finally resuspended in NK cell expansion media containing 10% autologous plasma and 700 IU/ml of IL-2 and with or without OK432. Suspended PBMCs were seeded at a density of 1 × 106/ml as per the protocol described by us earlier [24] and cells were cultured for the period of 14 days in a cGMP complaint cell processing room. The cell expansion was documented in terms of quantity by manual counting under an inverted phase contrast microscope at various intervals.
The expanded NK cell phenotype and purity was assessed by employing markers via CD56-PE and CD16-PC7 (Beckman Coulter Inc., USA). Immunostaining was performed as described in manufacturer's instruction. Stained cells were re-suspended in PBS and analyzed by using flow cytometry (FC- 500 Beckman Coulter, USA). The acquired data was analyzed using CXP software provided by the manufacturer.
The Breast cancer cell line (MDA-MB-231) was purchased from American Type Culture Collection (ATCC) USA Cells were cultured according to the recommendation of the supplier, in DMEM containing 10% FBS (Invitrogen, BRL, Burlington, ON, Canada). Cells were cultured without any stimulatory supplements in a humidified incubator at 37 ℃ with 5% CO2. Upon 70-80% confluence, the adhered cells were passaged by trypsinization (0.25% trypsin-EDTA, Invitrogen, BRL, Burlington, ON, Canada) and consumed for downstream experiments.
Total RNA was extracted from NK cells with and without OK432 supplement by using RNeasy Mini Kit (QIAGEN). The preparation of the first-strand cDNA was conducted following the instruction of the QuantiTect (Qiagen). RT-PCR analysis was performed using the QuantiTectRev.Transcription Kit (QIAGEN, Valencia, CA, USA). Gene of interest was obtained using primers synthesized from AIT biotech (Singapore) as shown in Table 1. Gene expression was quantified by ImageJ software and the gene expression was normalized with housekeeping gene, GAPDH.
Table 1: List of primers used for RT-PCR. View Table 1
The target of MDA-MB-231 cells were seeded in 96-well plates at a density of 3 × 103 cells/well and incubated for 24 h. The media was removed and washed with PBS twice, then the effector cells (NK cells) with and without OK432 supplement were seeded at different ratio (E:T) [ 1:1, 2:1, 5:1, 10:1 and 20:1] for different time periods (4 h and 24 h). MTT test was then performed after the plates were centrifuged 1500 rpm for 5 mins. Cytotoxicity was calculated using the formula: (Test OD - effector control OD)/ target control OD × 100. Triplicate experiments were performed and average values with mean ± SEM were represented.
All data were statistically analyzed using Student t-test. Data were presented as the mean ± standard deviation for cytotoxicity and RT-PCR assay P < 0.05 was considered to be statistically significant.
NK cell obtained from PBMC fractions of whole blood derived from healthy donors were cultured and expanded with and without the presence of supplement (OK432) for the period of 14 days. Figure 1 shows that the mean average cell number was increased from Day 7 and after that the cell growth was exponential increased in both groups until cells were harvested. Maximum numbers of cells were achieved on day 14 in both groups; which represent mean average of 600 ± 05 million (with OK432) and 589 ± 0.7 million (without OK432). These result indicated that OK432 do not influence on NK cell proliferation.
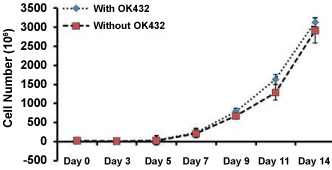 Figure 1: NK cell growth kinetics. Actual number of peripheral blood derived NK cells were counted over the period of expansion by using dye exclusive method. Three consecutive experiments were performed form three different donors.
View Figure 1
Figure 1: NK cell growth kinetics. Actual number of peripheral blood derived NK cells were counted over the period of expansion by using dye exclusive method. Three consecutive experiments were performed form three different donors.
View Figure 1
Next, we investigated whether OK432 influence on NK cell phenotyping or not. Our flow cytometry analysis revealed that the average expression of CD56 and CD16 was significantly increased (83.8 ± 0.4 and 65.5 ± 0.6, respectively) in cells treated with OK432. Whereas, cells cultured without OK432 could exhibit lower expression of CD56 and CD16, 65.3.8 ± 0.2 and 49.6 ± 0.3, respectively (Figure 2). In addition to that the mRNA expression of Perforin and NKG2D was significantly increased in cell cultured with OK432 than that of without OK432 (Figure 3). Taken together, our results demonstrated that OK432 potentially regulate the NK cells function by altering the cytotoxic gene and protein expression.
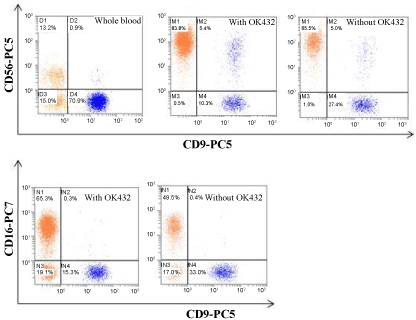 Figure 2: Immunophenotyping of expanded NK cells. Flow cytometry analysis represent the percentage of potential and activated NK cell population (CD56+ and CD16+) on before and after expansion in the presence or absence of OK432.
View Figure 2
Figure 2: Immunophenotyping of expanded NK cells. Flow cytometry analysis represent the percentage of potential and activated NK cell population (CD56+ and CD16+) on before and after expansion in the presence or absence of OK432.
View Figure 2
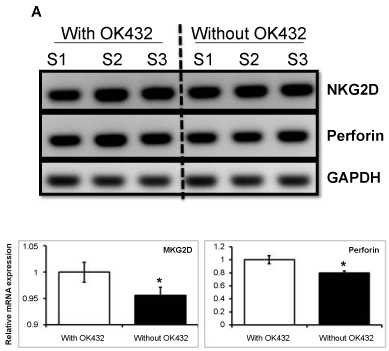 Figure 3: Semi-quantitative PCR for NK cell potency. NK cells activity and functionality was assessed as based on the expression of NKG2D and Perforin, respectively. Three consecutive experiments were performed form three different donors. Electrophoresis gel images and their respective densitometry clearly revealed that OK432 significantly up-regulate the NKG2D receptor and Perforin on NK cells. The variation within each set of triplicates is shown with mean of SD ± *P < 0.05 (n = 3).
View Figure 3
Figure 3: Semi-quantitative PCR for NK cell potency. NK cells activity and functionality was assessed as based on the expression of NKG2D and Perforin, respectively. Three consecutive experiments were performed form three different donors. Electrophoresis gel images and their respective densitometry clearly revealed that OK432 significantly up-regulate the NKG2D receptor and Perforin on NK cells. The variation within each set of triplicates is shown with mean of SD ± *P < 0.05 (n = 3).
View Figure 3
It has been demonstrated earlier that the in-vitro culture process activates NK cells and increases their cytotoxicability compared to freshly isolated cells. However, our study aimed to assess the cytotoxicability of NK cells expanded with and without OK432 against MDA-MB-231. Our cytolysis analysis shown that both OK432 primed and un-primed NK cells absolutely (100%) killed all target cells after 4 h co-culture at the E:T ratio of 20:1. Whereas, there was no significant variation noted in between with and without OK432 treated NK cells against MDA-MB-231. Cytolysis of with and without OK432 treated NK cells on cancer cells was 79 ± 4% vs. 60 ± 1.7%, 55 ± 6% vs. 42 ± 4%. 37 ± 3.8 vs. 36 ± 3%, and 27 ± 3% vs. 24 ± 3.5%, at the E:T ratio of 10:1, 5:1, 2:1 and 1:1, respectively. Furthermore, we curious to know that whether NK cell mediated cancer cell lysis was increased on time dependent manner. In order to that both OK432 primed and un-primed NK cells were co-cultured with cancer cells for 24 h at different E:T ratio. Our cytotoxicity analysis revealed that overall cancer cell lysis was increased in both OK432 treated and untreated NK cells at different E:T ratio as compared to 4 h incubation. Nevertheless, complete cancer cell cytolysis was achieved at 10:1 ratio in OK432 whereas 80% of cytolysis was achieved in untreated NK cells. Noteworthy, there was significant variation in cancer cell lysis was noted in between OK432 treated and untreated NK cells at the ratio of 5:1, 2:1 and 1:1. In comparison to OK432 untreated NK cell, OK432 treated NK cells shows increased cytolysis potential for 24 h incubation (Figure 4a). Our microscopic examination also confirms our cytolysis assay that NK cell mediated cytolysis was increased on dose and time dependent manner (Figure 4b).
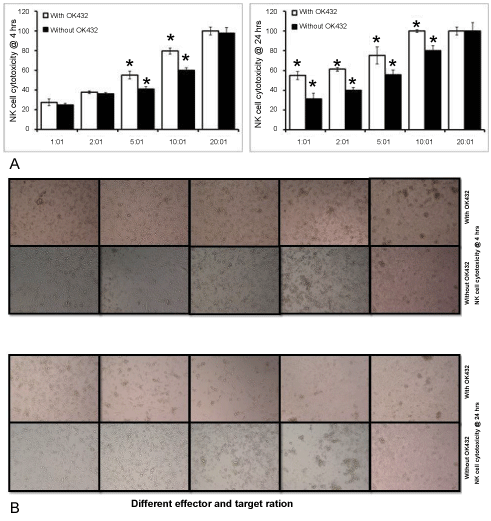 Figure 4: Cellular cytotoxicity of expanded NK cells. a) Killing efficacy of expanded NK cells in the presence or absence of OK432 towards breast cancer cell line at different E:T ratio for 4 h and 24 h; b) Microscopic images of NK cell cytotoxicity of breast cancer cells at different E:T ratio for 4 h and 24 h. The variation within each set of triplicates is shown with mean of SD ± *P < 0.05 (n = 3). The cytotoxicity was better in cells treated with OK432 and all the target was lysed at 10:1 ration within 24 h.
View Figure 4
Figure 4: Cellular cytotoxicity of expanded NK cells. a) Killing efficacy of expanded NK cells in the presence or absence of OK432 towards breast cancer cell line at different E:T ratio for 4 h and 24 h; b) Microscopic images of NK cell cytotoxicity of breast cancer cells at different E:T ratio for 4 h and 24 h. The variation within each set of triplicates is shown with mean of SD ± *P < 0.05 (n = 3). The cytotoxicity was better in cells treated with OK432 and all the target was lysed at 10:1 ration within 24 h.
View Figure 4
Natural killer cells (NK cell) are the first line of defense system against virally infected cells and transformed cells. It is a type of innate immune cell and plays a major role in cancer immunosurveillance [25]. However, NK cells activity is suppressed when malignant cells secrete an immunosuppressive microenvironment and result in exhaustion from immune system and producing tumor secreting factors such as soluble MICA, MICB, and vesicles containing ULBP3 [26]. These factors cause decreased expression of NK cell-activating receptors while increasing the expression of inhibitory receptors [27]. As NK cells represent a small fraction of peripheral blood (PB) and freshly isolated NK cells from healthy donor/cancer patients always show low cytotoxicity against tumor cells [28]. Therefore, ex vivo activation and expansion of NK cells is crucial to achieve better cell lysis in adoptive cancer immunotherapy. Several methods for NK cells expansion using IL-2, IL-15, and OKT3 have been reported [29,30]. It has been shown that NK cells that had been stimulated with Hsp70 protein/peptide plus low dose IL-2 significantly up-regulated the production of granzyme B [31]. Recently, we reported that OK432 is a potential mytogen to activate and expansion of the NK cells and it showed a better tumor killing activity on colorectal cancer patients [24]. In the current study, we have investigated in-vitro cytolytic ability of NK cells treated with and without OK432 on breast cancer cell line.
Our results clearly showed that NK cells were shrilly expanded with or without the presence of OK342. However, the presence of OK432 significantly promoted the expression of CD56 and CD16. To agree our results, Chu, et al. [32] proven that NK cell proliferation and surface marker CD56 and CD16 expression were higher with cells cultured with OK432 [32]. Studies also showed that Furthermore our data revealed that the expanded NK cells expressed NKG2D activation receptor and Perforin. Our cytotoxicity analysis showed that OK432 promoted perforin-mediated NK cell cytotoxicity which may be dependent on NKG2D receptor of NK cells. Guma, et al. [33] also reported that NK cell-mediated lysis was dramatically dropped by blocking NKG2D receptor. These observations apparently demonstrated that NK cells are most aggressively eliminate the cancer cell through NKG2D receptor via increased MAPK pathway (JNK) phosphorylation. Perforin released in the target cells polymerizes and forms the pores and facilitating the entry of granzymes into the target cell and leading to induction of apoptosis of target cells [34,35].
In conclusion, the ex vivo expanded NK cells pre-activated with OK432, might be a useful cellular source for NK cell adoptive immunotherapy for the patients with breast cancer.
R&D Fund from Ministry of Energy, Science, Technology, Environment and Climate Change (305/CIPPT/613240) for supporting this research. OK432 was sponsored by Chugai Pharmaceutical Co., Ltd Japan.