We compared analgesic efficacy and safety of sub-dissociative dose ketamine (SDK) to morphine for managing acute traumatic pain in geriatric emergency department (ED) patients.
A subset of geriatric patients from a randomized double-blind trial experiencing moderate to severe acute traumatic pain who received SDK at 0.3 mg/kg or morphine at 0.1 mg/kg by short intravenous infusion over 15 minutes was analyzed at 15, 30, 60, 90, and 120 minutes post-medication administration. Primary outcome was reduction in pain at 30 minutes. Secondary outcomes included adverse effects and rates of rescue analgesia.
Twelve patients (5 in the SDK group and 7 in the morphine group) were eligible for analysis. The change in mean pain scores was not significantly different in SDK and morphine groups: 8.6 versus 9.1 at baseline and 5.8 versus 5.9 at 30 minutes. Patients in SDK group had a greater percentage change in pain reduction from the baseline in comparison to morphine at the 15-minute mark (48.8% decrease versus 30.8% decrease). In the morphine group more, patients experienced dizziness (57% vs. 20%) and fatigue (71% vs. 40%), and required more rescue analgesia at 60, 90 and 120 minutes (14% vs. 0%; 14% vs. 0%, and 29% vs. 0%, respectively).
SDK at 0.3 mg/kg over 15 minutes provided analgesia comparable to morphine for short-term treatment of acute pain with a reduced need for rescue analgesia for up to two hours and minimal rates of rates of psychoperceptual adverse effects.
Ketamine, Geriatric, Analgesia, Emergency department, Trauma
Management of acute traumatic pain in elderly patients in the Emergency Department is complicated and therefore frequently leads to suboptimal pain relief [1-6]. Age-related changes in drug absorption and metabolism, reduced clearance of medications, and multiple drug-drug interactions put elderly patients at significant risk of analgesic-related adverse effects and treatment failures. The administration of opioid analgesics to elderly patients frequently leads to hemodynamic instability with increased morbidity, hypotension, and respiratory and central nervous system depression [6,7]. Provision of effective, timely and safe analgesia for geriatric patients presenting to the Emergency Department (ED) with acute traumatic injuries results in significant reduction of patients' pain, improvement in the diagnostic workup, alleviation of anxiety and fear, and improved satisfaction of patients, their families, and staff members [7].
Data shows that elderly patients are less likely to receive opioid analgesia or receive an appropriate dose of opioids both in the ED and on discharge in comparison to their younger counterparts [6,7]. Based on aforementioned challenges of managing geriatric pain in the ED, ED clinicians might consider exploring the utilization of non-opioid analgesics that can provide comparable or better pain relief than opioids and lower rates of serious adverse effects. One of the potential analgesics to serve this purpose is sub-dissociative dose ketamine (SDK).
Ketamine is a non-competitive N-methyl-D-aspartate (NMDA)/glutamate receptor complex antagonist that provides analgesia by reducing central sensitization, "wind-up" phenomenon, and hyperalgesia at the level of the spinal cord and central nervous system [8-10]. The use of ketamine in full dissociative doses for induction of anesthesia and procedural sedation in the ED frequently leads to development of emergence phenomena and cardiovascular stimulation which brings significant degree of reluctance amongst clinician to use ketamine outside of familiar indications [9,10]. However, when ketamine is given in sub-dissociative (low, analgesic) doses, the adverse effects are short-lived and modest in intensity, not associated with cardiovascular or respiratory compromise, and primarily involve neuro-psychiatric disturbances [11,12]. Sub-dissociative dose ketamine with a dosing range of 0.1-0.3 mg/kg provided effective pain relief for patients with acute traumatic and non-traumatic pain, chronic non-cancer and cancer pain, and in patients with opioid-tolerant pain in pre-hospital settings and in the ED [11-15]. Two commonly utilized strategies of SDK administration in our ED include an intravenous push (IVP) dose (over 2-5 minutes) that is associated with high rates of bothersome psychoperceptual side effects (feeling of unreality and dizziness), or short infusion (SI) given over 15 minutes with reduced rates of psychoperceptual side effects and preserved analgesic efficacy [11]. To our knowledge, there is no data that evaluated SDK role in managing a variety of acute traumatic conditions in geriatric patients in the ED.
In our study we describe a comparative change in pain score and rates of adverse effects in the subset of geriatric patients suffering from acute traumatic painful conditions who received either a sub-dissociative dose ketamine at 0.3 mg/kg via short infusion over 15 minutes, or morphine at 0.1 mg/kg administered over 15 minutes.
This subset of geriatric patients suffering from acute traumatic injuries in the ED was a part of a large prospective randomized double-blind clinical trial comparing the analgesic efficacy and safety of intravenous SDK to intravenous morphine both administered as a short infusion (over 15 min) for acute pain in elderly ED patients that was published in American Journal of Emergency Medicine [16]. This trial that included a total of 60 patients was conducted in a 711-bed community teaching hospital with an annual ED census of more than 120,000 visits. The study included patients aged 65 and older who presented to the ED with acute abdominal, flank, back, or musculoskeletal pain with an initial pain score of 5 or more on a standard 11-point (0 to 10) numeric rating scale and required opioid analgesia [17,18]. Exclusion criteria included allergy to morphine or ketamine, altered mental status, weight less than 40 kg or greater than 115 kg, unstable vital signs (systolic blood pressure < 90 or > 180 mmHg, pulse rate < 50 or > 150 beats/min, and respiration rate < 10 or > 30 breaths/min), and past medical history of acute head or eye injury, seizure, intracranial hypertension, severe chronic obstructive pulmonary disease, chronic pain, renal or hepatic insufficiency, alcohol or drug abuse, psychiatric illness, or recent (4 hours before) opioid use. The primary outcome of the trial included a comparative reduction of pain scores on numeric rating pain scale (NRS) between recipients of SDK and morphine at 30 minutes. The secondary outcomes consisted of a need for rescue analgesia at either 30 or 60 minutes and adverse events in each group. With respect to unique adverse effects in SDK group, we used Side Effect Rating Scale for Dissociative Anesthetics (SERSDA) and Richmond Agitation Sedation Scale (RASS) [19,20]. SERSDA Scale includes fatigue, dizziness, nausea, headache, feeling of unreality, changes in hearing, mood change, general discomfort, and hallucinations with severity of each graded by patients on a five-point scale, with "0" representing the absence of any adverse effects and "4" representing a severely bothersome side effect. RASS evaluates the severity of agitation and/or sedation in accordance to the nine-point scale with scores ranging from "-4" (deeply sedated) to "0" (alert and calm) to "+4" (combative). This study was approved by the Maimonides Medical Center institutional review board and registered with clinicaltrials.gov (NCT02673372). The study was conducted and reported according to the Consolidated Standards of Reporting Trials Group [21].
The results of the original trial demonstrated that SDK was as effective as morphine in relieving pain at 30 minutes but with significantly higher rates in overall adverse effects at 15 minutes post-administration (86.7% vs. 46.7%) and at 30 minutes (73.3% vs. 36.7%) but with reduced need for rescue analgesia at 60, 90 and 120 minutes. (6% vs. 24%).
The present research project is aimed to analyze a comparative change in pain scores, rates of side effect and a need for rescue analgesia in the subset of geriatric patients receiving SDK or morphine for acute traumatic pain.
Twelve patients (5 in SDK group and 7 in morphine group) were eligible for subgroup analysis. The patients' mean age was 79 and 76 years with male patients comprising 40% and 14% in each group respectively. There were no differences between the groups in terms of baseline vital signs and pain scores. However, more patients in the morphine group had acute traumatic pelvic fracture (71%) as a source of pain (Table 1).
Table 1: Baseline patient characteristics. View Table 1
All patients showed significant reductions in mean pain score at 15-120 minutes in comparison to baseline (Table 2). At 15-minutes, the SDK group demonstrated a greater percentage change in pain score from the baseline in comparison to morphine (48.8% decrease versus 30.8% decrease). At 30 minutes, the percentage change in pain score was essentially the same (32.6% versus 35.2%). The box plots of the difference show a similar pattern of central tendency and dispersion. A comparison of the pain scores over time demonstrates similar percentage change in a pain score between two groups with exception of the 15-minute time frame where the SDK group had a greater percentage change in mean pain score (Figure 1). In addition, more patients in the SDK group had complete resolution of pain at 30 minutes (20% vs. 0%).
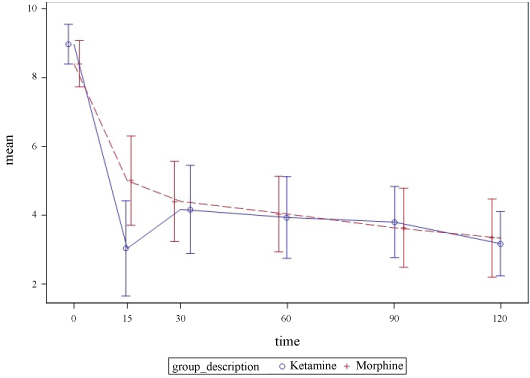 Figure 1: Reported pain numeric rating score with 95% CI bars. View Figure 1
Figure 1: Reported pain numeric rating score with 95% CI bars. View Figure 1
Table 2: Pain trends. View Table 2
None of the patients in each group required rescue analgesia at 30 minutes. However, at 60-120 minutes, more patients in the morphine group required rescue analgesia (4 patients vs. 0 patients) with percent difference of 14%, 14% and 29% respectively (Table 2).
With respect to rates and severity of adverse effects in the SDK group on the SERSDA scale, 2 patients experienced side effects (dizziness, fatigue, and change in vision) that were weak-to-modest in intensity. One patient experienced a bothersome headache at 15 minutes (Table 3) and one patient reporting moderate sedation according to the RASS scale (Table 4). By comparing most common adverse effect between two groups, more patients in morphine group experienced dizziness (57% vs. 20%) and fatigue (71% vs. 40%) (Table 5).
Table 3: Severity of SERSDA. View Table 3
Table 4: Patients reporting agitation or sedation according to RASS. View Table 4
Table 5: Rates of adverse effects & intervention. View Table 5
No serious or life-threatening adverse events occurred in either group. There were no changes in vital signs that were clinically concerning. Most adverse effects were transient and did not require treatment, with exception of one patient in the SDK group who required supplemental oxygen for correction of brief desaturation at 15 minutes (Table 6).
Table 6: Report of any intervention. View Table 6
The small number of geriatric patients suffering from acute traumatic injuries in this case series does not allow us to make any definitive conclusion on the true analgesic efficacy and safety of SDK. As these patients had only acute musculoskeletal injury, it limits our ability to extrapolate the results of our analysis to other traumatic injuries.
SDK remains an attractive analgesic modality for patients presenting to the ED with a variety of acute traumatic and non-traumatic painful conditions. However, the published data on its safety and efficacy is limited to patients younger than 65 years of age. Possible reasons for limited utilization of SDK in geriatric ED patients include a lack of experience, reluctance of ketamine utilization due to emergence phenomenon, hyperdynamic vital signs, increased intracranial and intraocular pressure, administrative and regulatory concerns as well as off-label use.
The results of our subset analysis of geriatric patients presenting to the ED with acute traumatic musculoskeletal pain and receiving SDK at 0.3 mg/kg dose over 15 minutes demonstrated analgesic efficacy comparable to morphine for up to 120 minutes, greater improvement in pain score at 15 minutes, and diminished need for rescue analgesia up to 120 minutes post-administration. In addition, SDK group had less patients experiencing dizziness and fatigue, and unique to dissociative anesthetic, small overall percentage of patients (21%) experiencing psychoperceptual side effects of weak to-moderate intensity. These findings are consistent with the findings of the original GERIKET trial, where more patient in the SDK group had larger decrease in pain scores at 15 minutes.
We believe that there is great need for further prospective randomized trials that will compare different dosing regimens and duration of infusions to find an optimal dosing regimen with a good balance of analgesic efficacy and rates of side effects.
In conclusion, our sub-group analysis of geriatric trauma patients demonstrated that sub-dissociative dose intravenous ketamine administered at 0.3 mg/kg over 15 min provides analgesic efficacy comparable to intravenous morphine for short-term (up to 120 minutes) treatment of acute moderate-to- severe pain in geriatric ED patients with minimal rates of weak-to-modest intensity psychoperceptual adverse effects.
None.
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors have no independent disclosures or conflicts of interest.
Study concept and design: Motov
Acquisition, analysis, or interpretation of data: All authors
Statistical analysis: Likourezos, Flom
Drafting of the manuscript: Motov, Drapkin, Fassassi
Critical revision of the manuscript for important intellectual content: Motov, Marshall, Simon
Study supervision: Motov, Likourezos.