Gas gangrene is a rare, life-threatening deep skin infection typically related to contaminated wounds, although it may occur without injury. Non-traumatic gas gangrene due to Clostridium spp. is most commonly caused by Clostridium septicum. We present the case of a patient who expired of atraumatic gas gangrene due to Clostridium septicum. C. septicum infection is rapidly progressive and is associated with colorectal cancer and diabetes mellitus. We emphasize that it is important to diagnose and treat patients with gas gangrene by early recognition and aggressive debridement.
Non-traumatic gas gangrene, Clostridium septicum, Debridement
Necrotizing soft tissue infection is a serious bacterial infection involving dermis, subcutaneous tissue, fascia, or muscle. It causes significant morbidity and is associated with a relatively high mortality rate ranging between 20% and 79% [1-3]. Necrotizing soft tissue infection can be classified by cause of microbial infection. Approximately 55% to 75% of all infections result from type I infection [4]. Type I infections are polymicrobial and the common source of microbes are a combination of gram-positive cocci, gram-negative rods, and anaerobes. Less commonly, the infection might be caused by Bacteroides or Clostridium. Type II infections are monomicrobial such as Staphylococcus, Streptococcus and Clostridium spp. Clostridium spp. is approximately 10% of cases of necrotizing soft tissue infection [5]. Clostridial soft tissue infections, traditionally known as gas gangrene present with the rapid onset of severe pain and the progressive invasion of healthy muscle tissue. Among the patients with gas gangrene only 15% of them were given a diagnosis upon admission [6]. Gas gangrene has two major presentations: traumatic and spontaneous. Traumatic gas gangrene is most commonly caused by Clostridium perfringens. Traumatic injury accounts for about 70% of gas gangrene cases and about 80% of these are caused by C. perfringens [7]. Less than 10% of gas gangrene occurs spontaneously in the absence of trauma [8]. Spontaneous gas gangrene is most commonly caused by Clostridium septicum [9]. In one report, only 21 cases were documented between 1900 to 1985; in a second series of over 20,000 autopsies, only four cases were found [10]. The incidence of Clostridium septicum bacteraemia in England and Wales has been reported to be 0.4-1.0 cases per million population [11] but cases of Clostridium septicum myonecrosis are even less common than this. There is paucity in literature on patients with non-traumatic gas gangrene due to C. septicum. The objective of this report is to promote awareness in the emergency medical care setting of patients with non-traumatic gas gangrene due to C. septicum.
A 73-year-old Japanese man with well controlled type II diabetes mellitus and hypertension presented to our emergency department with a complaint of a 2-day history of left upper arm pain and a 4-hour history of left lower back pain. He had no history of trauma or surgery. This patient had had diabetes mellitus since 13 years prior to admission. He was treated with α-glucosidase inhibitor (Voglibose 0.6 mg/day). This is the only information we had since he had been followed up at a different clinic. He expired so rapidly, and we do not have further information at this point. He did not have apparent complications due to diabetes mellitus such as retinopathy or visual changes, and neuropathy. His renal function was abnormal on admission most likely due to sepsis. His HbA1c was 6.9% on admission. His initial vital signs were unremarkable except for the respiratory rate: Blood pressure 105/73 mmHg; Pulse rate, 89/min, regular; Respiratory rate, 26/min; Body temperature, 36.2 ℃. His mental status was not altered (Glasgow Coma Scale 15). The left lower back showed severe pain, although there was no skin rash. One hour later the left lower back showed a wide range of discoloration, and palpable gas appeared under the skin and his back pain worsened. There was no tenderness or warmth. Other physical examination findings were unremarkable. Initial laboratory findings showed that white blood cell count was 13,000/μL and 60% of neutrophils were band form. Creatine phosphokinase was 2,569 U/L. The radiographs of the trunk showed extensive gas in the soft tissues of the left thoracic dorsal region extending to the pelvis (Figure 1). There were similar findings in the computed tomography of the chest and abdomen without contrast material (Figure 2). One hour after admission, a diagnosis of necrotizing soft tissue infection was made. Broad-spectrum antimicrobial agents including vancomycin 0.5 g every 12 hours (Day 1 - Day 3), meropenem 1 g every 12 hours , clindamycin 600 mg every 8 hours were urgently administered together with crystalloid rehydration and vasopressors. However his skin discoloration region gradually expanded by the hour. The patient deteriorated rapidly and 7 hours after admission he demonstrated septic shock. The patient was taken immediately to the operating room for urgent surgical debridement. The range of myonecrosis with gas formation was too extensive for adequate debridement. After the operation, intensive care was provided to treat multi organ dysfunction. The control of the infection source was not successful and the patient died of overwhelming sepsis on day 6 of admission. The Gram stain of the necrotic tissues revealed an abundance of spore-forming gram-positive bacilli. The infecting organism was isolated but not identified by routine automated methods of tissue samples and blood cultures. Genetic sequence analysis identified the organism as Clostridium septicum allowing the final diagnosis of Clostridial myonecrosis, i.e. gas gangrene, to be made. An autopsy discovered a previously undiagnosed advanced cancer of the ascending colon in the patient. Grossly, tumor was type 2 and 2 cm in diameter. Histologically tumor was well to moderately differentiated tubular adenocarcinoma and invaded muscularis propria. He had never received an examination by colonoscopy. There was no metastasis finding in other organs.
 Figure 1: The chest X-ray (anteroposterior view) showed subcutaneous gas formation in the left lateral thoracic. View Figure 1
Figure 1: The chest X-ray (anteroposterior view) showed subcutaneous gas formation in the left lateral thoracic. View Figure 1
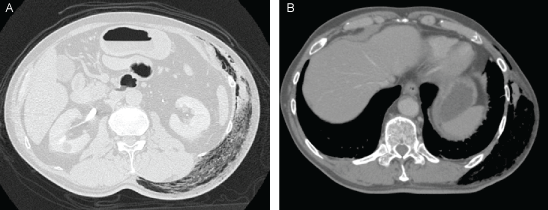 Figure 2: A.B) The computed tomography of the chest and abdomen without contrast material showed subcutaneous gas formation extending to the pelvis. View Figure 2
Figure 2: A.B) The computed tomography of the chest and abdomen without contrast material showed subcutaneous gas formation extending to the pelvis. View Figure 2
We reported on a patient of non-traumatic gas gangrene due to C. septicum with colon cancer revealed by autopsy.
Gas gangrene is a rare, life-threatening infection of deep skin and soft tissues. Gas gangrene is typically associated with contaminated wounds. It may also occur in a state of immune-suppression such as malignancy, even when there has been no injury [12]. Clostridium septicum accounts for 4% to 20% of Clostridial infection and known to cause non-traumatic gas gangrene [13]. C. septicum is part of normal gastrointestinal tract flora but when infection occurs is often fatal, especially in the diabetic population [14]. C. septicum is capable of infecting normal tissue and thought to gain access to the bloodstream through the gastrointestinal mucosa [15]. Both Gas gangrene due to C. septicum and necrotizing soft tissue infection of Group A Streptococcus (GAS) have similar clinical findings and rapid clinical courses. It is difficult to differentiate between these two infections. However compared with GAS, patients with C. septicum infection are likely to have colorectal or haematological cancer [16]. It is necessary to rule out the possibility of these infections that a comprehensive approach focusing on a careful history taking and repeated physical examinations in order to search changes in skin findings. In addition, exploratory incision and microscopic analysis such as Gram stain of the tissues can confirm the diagnosis and associated complications [17]. In our case, colon cancer revealed by autopsy confirmed the diagnosis of gas gangrene due to C. septicum. When gas gangrene of the trunk progresses to a wide range of skin necrosis, extensive debridement usually becomes difficult. Although initial laboratory and imaging findings may show nonspecific signs, it is critically important that this highly fatal condition is considered early in patients with gas gangrene especially when onset occurs in the trunk [18].
Treatment of spontaneous gas gangrene consists of emergent surgical debridement of the entire affected area and parenteral antimicrobial therapy. Extensive debridement of the affected area including “second look” is critical for saving lives [5]. It must be performed in a timely fashion and otherwise these kinds of patients with gas gangrene or necrotizing deep skin soft tissue infections have very high mortalities [19]. Empiric treatment with effective antimicrobial agents is another crucial part of the management of the patients with gas gangrene. The therapy should include broad spectrum antimicrobial agents covering not only Clostridium spp. but also other aerobic and anaerobic microbial species. Until culture results are available, it is reasonable to cover Staphylococcus aureus and Streptococcus spp. Gram negative bacilli including Enterobacteriaceae, and Pseudomonas aeruginosa, and anaerobes such as Bacteroides spp. In the management of patients with deep skin and soft tissue infections and gas gangrene, clindamycin has a very important role to inhibit toxin production by pathogens such as Group A Streptococcus and Clostridium spp. [20].
Table 1 shows recommended antimicrobial therapy classified by pathogens of necrotizing soft tissue infection. For Gram positive coverage, vancomycin, linezolid, and daptomycin can be used because of concern for methicillin resistant Staphylococcus aureus infection, especially in intravenous drug users [4]. For Gram negative coverage, anti-pseudomonal agents can be used empirically until culture results are available if the patients are critically ill. For anaerobic coverage, combination of β-lactams and Beta-lactamase inhibitors such as piperacillin/tazobactam, or carbapenems such as meropenem, with metronidazole can cover Bacteroides fragilis group as part of the mixed causative organisms. For suppression of the toxin production, lincosamides such as clindamycin should be administered for better outcomes. Definitive antimicrobial therapy with penicillin and clindamycin is recommended for treatment of Clostridial myonecrosis [21]. Early-awareness in order to make a prompt diagnosis and urgent and thorough surgical debridement is essential to improve survival [22]. A multidisciplinary team approach by multiple departmental doctors such as general physicians, orthopedic surgeons, dermatologists, pathologists, co-medical staff such as nurses, pharmacists, and laboratory technicians is necessary to manage these patients because those infections require intensive care pre- and post-operatively [23].
Table 1: Recommended antimicrobial therapy classified by pathogens of necrotizing soft tissue infection [4,20,21]. View Table 1
In conclusion, a patient of non-traumatic gas gangrene due to C. septicum with colon cancer revealed by autopsy was reported. Emergency physicians should be aware of the possibility of gas gangrene in patients with severe pain even if skin color change is not yet evident. If necrotizing soft tissue infections including gas gangrene are suspected, appropriate management by timely surgical debridement and broad-spectrum antimicrobial therapy should be provided in order to improve the clinical outcome.
No authors have any conflicts of interest.
Ethical approval was not required in this report.