Described herein the case of a 55-year-old man diagnosed with advanced heart failure. He had a history of ischemic heart disease leading to moderate left ventricular systolic disfunction, symptomatic for effort dyspnoea despite optimal medical therapy. After coronary angiography and right heart catheterization (showing basal pulmonary capillary wedge pressures - PCWP - 20 mmHg), the patient underwent percutaneous inter-atrial shunt therapy (IAST) through the opposition of an Atrial Flow Regulator (AFR) device (Occlutech™) to reduce left atrial pressures (LAP) and theoretically ameliorate his symptoms. LAP was acutely reduced after the procedure and the patient developed a significant clinical and functional improvement at 3 months.
Heart failure, Atrial flow regulator, Inter-atrial shunt therapy, Inter-atrial shunt devices, Left atrial pressure
Heart failure (HF) is a clinical syndrome characterized by typical symptoms (e.g. dyspnoea, orthopnoea or fatigue) and signs (pulmonary crackles or peripheral oedema) caused by a structural and or functional cardiac abnormalities, resulting in a reduced cardiac output and/or elevated intracardiac pressures [1]. HF is an irreversible, progressive disorder that affects more than 23 million people worldwide, including 1-2% of the whole adult population [2,3]. Coronary artery disease is a common cause of HF and both conditions share several risk factors such as obesity, arterial hypertension and diabetes mellitus [4]. HF is a leading cause of hospitalization and it confers a substantial burden to the healthcare system: in the U.S. the total medical costs for patients with HF are expected to rise from US$20.9 billion in 2012 to $53.1 billion by 2030 [4]. Both, morbidity and mortality are increased and some prognostic models have been proposed in order to predict patients’ clinical outcomes [4,5]. Patients with HF and reduced ejection fraction (HFrEF) usually benefit from drug therapy consisting of angiotensin-converting-enzyme inhibitors, angiotensin receptor-neprilysin inhibitors (ARNI), beta-blockers, mineralocorticoid receptor antagonists, inhibitors of sodium-glucose co-transporter-2 (SGLT2) and, in selected cases, from non-pharmacological approaches (e.g. cardiac resynchronization therapy in subjects with left ventricular ejection fraction (LVEF) ≤ 35% and complete left bundle branch block (LBBB) [1,6,7]. Instead, up to nowadays, there is no substantiated effective treatment for HF and preserved ejection fraction (HFpEF). Increased left atrial pressure (LAP) is thought to be the common final pathological way to cause decompensated HF symptoms, even in patients treated with optimal medical management [8]. Dorfs, et al. showed that pulmonary capillary wedge pressure (PCWP) at rest and an excessive rise of this parameter during physical exercise are associated with increased mortality in a group of 355 patients with HFpEF [9]. Inter-atrial shunt therapy (IAST) has been recently proposed as an innovative strategy to reduce LAP and PCWP in patients with advanced HF in order to ameliorate symptoms and improve functional status [1-12].
A 55-year-old man, hypertensive with type 2 insulin-dependent diabetes mellitus, dyslipidaemia and obesity (BMI: 33.96 kg/m2) underwent clinical evaluation because of more-than-3-month effort dyspnoea (NYHA III). His past medical history consisted of prior anterior ST-elevation myocardial infarction (STEMI) treated by percutaneous coronary intervention (PCI) with drug-eluting stent (DES) implantation on the left descending artery (LDA) and on the posterolateral artery (PLA). At follow-up, both DES resulted occluded while another PCI with DES implantation was performed on the obtuse marginal artery (OM1) due to a symptomatic critical CAD. A subsequent stress cardiac magnetic resonance (CMR) showed a moderate LV dysfunction (LVEF 40%) without viability of the left ventricular apex and the anterior wall. The patient was on optimal medical therapy including beta-blockers, ARNI, SGLT-2 inhibitor, diuretic, mineralocorticoid receptor antagonist). According to the recent 2021 ESC Guidelines on HF, the patient was diagnosed with advanced heart failure [1]: he presented typical symptoms of HF with persisting high N-terminal prohormone of Brain Natriuretic Peptide (NT-proBNP) and evident cardiac structural abnormalities; moreover, he had 4 episodes of pulmonary congestion requiring hospitalization, the last registered 6 months before, and developed a severe inability even to low-grade exercise. Due to this clinical scenario, the patient was admitted to our Cardiology Unit. Baseline trans-thoracic echocardiography confirmed left ventricle systolic impairment (LVEF 40%), but showed no right heart dysfunction nor severe pulmonary hypertension (e.g. pulmonary artery systolic pressure - PASP - ≥ 60 mmHg). Estimated ultrasonographic LAP at rest was 26 mmHg. Coronary angiography confirmed an occlusive in-stent restenosis on the LAD and a critical stenosis in the right coronary artery treated by DES implantation. Right heart catheterization reported mild pulmonary hypertension (PASP: 32 mmHg, diastolic pulmonary artery pressure - PAP: 24 mmHg, mean PAP: 27 mmHg) and increased PCWP at rest which was 20 mmHg. Based on these data the patient was considered a candidate for IAST using an 8-mm-diameter Atrial Flow Regulator device (Occlutech™). The procedure was successfully performed under general anaesthesia, through a hybrid fluoroscopic and transoesophageal echocardiographic guide. Procedural steps are described by Paitazoglou C, et al. [12] and are summarized in Figure 1. No complications were reported and the LAP has been acutely reduced following the generation of an iatrogenic inter-atrial shunt (final Qp/Qs 1.2) (Figure 2 and Figure 3). Patient was discharged 2 days after the procedures on dual antiplatelet therapy in association with beta-blocker, ARNI, diuretic, mineralocorticoid receptor antagonist, SGLT-2 inhibitor.
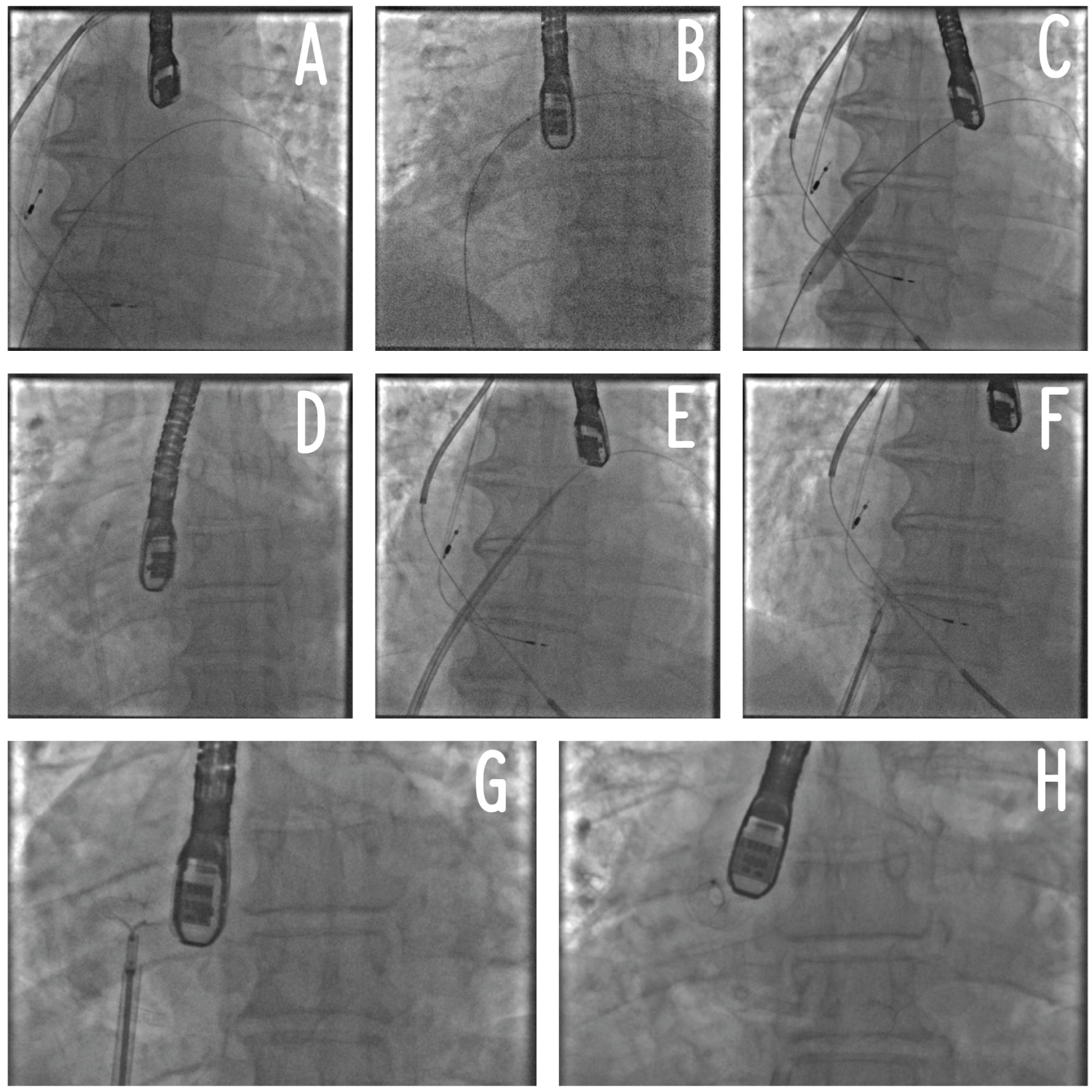 Figure 1: Procedural steps of AFR implantation. Panel A: positioning of a stiff wire in the left superior pulmonary vein; Panel B: atrial septostomy and septal dilatation (part 1); Panel C: septal dilatation (part 2); Panel D: crossing of the AFR delivery system through the septostomy inside the left atrium; Panel E: Advancement of the AFR delivery system inside the left atrium; Panel F: AFR initial release; Panel G: AFR traction test before complete release; Panel H: AFR complete release. AFR: Atrial Flow Regulator.
View Figure 1
Figure 1: Procedural steps of AFR implantation. Panel A: positioning of a stiff wire in the left superior pulmonary vein; Panel B: atrial septostomy and septal dilatation (part 1); Panel C: septal dilatation (part 2); Panel D: crossing of the AFR delivery system through the septostomy inside the left atrium; Panel E: Advancement of the AFR delivery system inside the left atrium; Panel F: AFR initial release; Panel G: AFR traction test before complete release; Panel H: AFR complete release. AFR: Atrial Flow Regulator.
View Figure 1
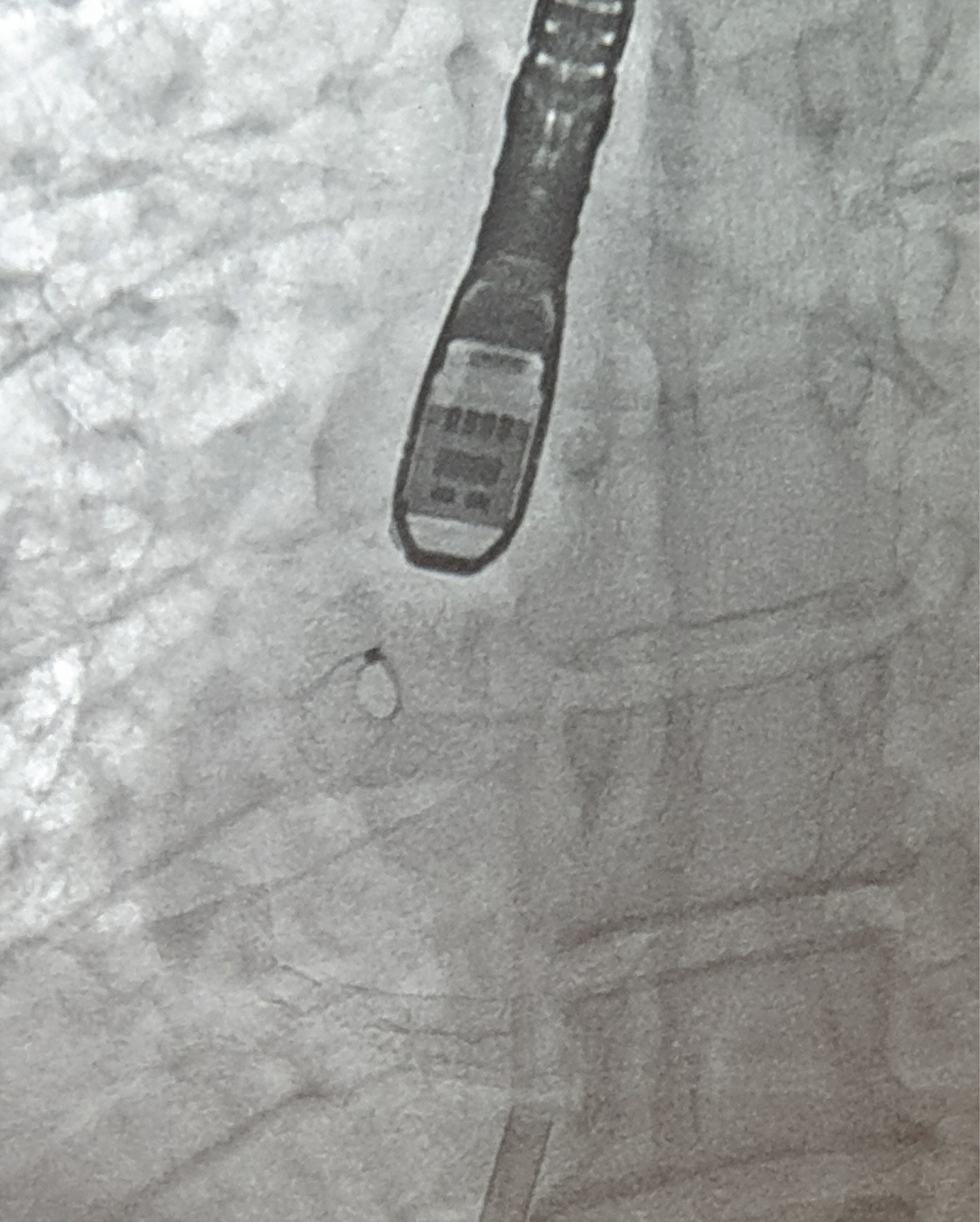 Figure 2: Fluoroscopic view of atrial flow regulator.
View Figure 2
Figure 2: Fluoroscopic view of atrial flow regulator.
View Figure 2
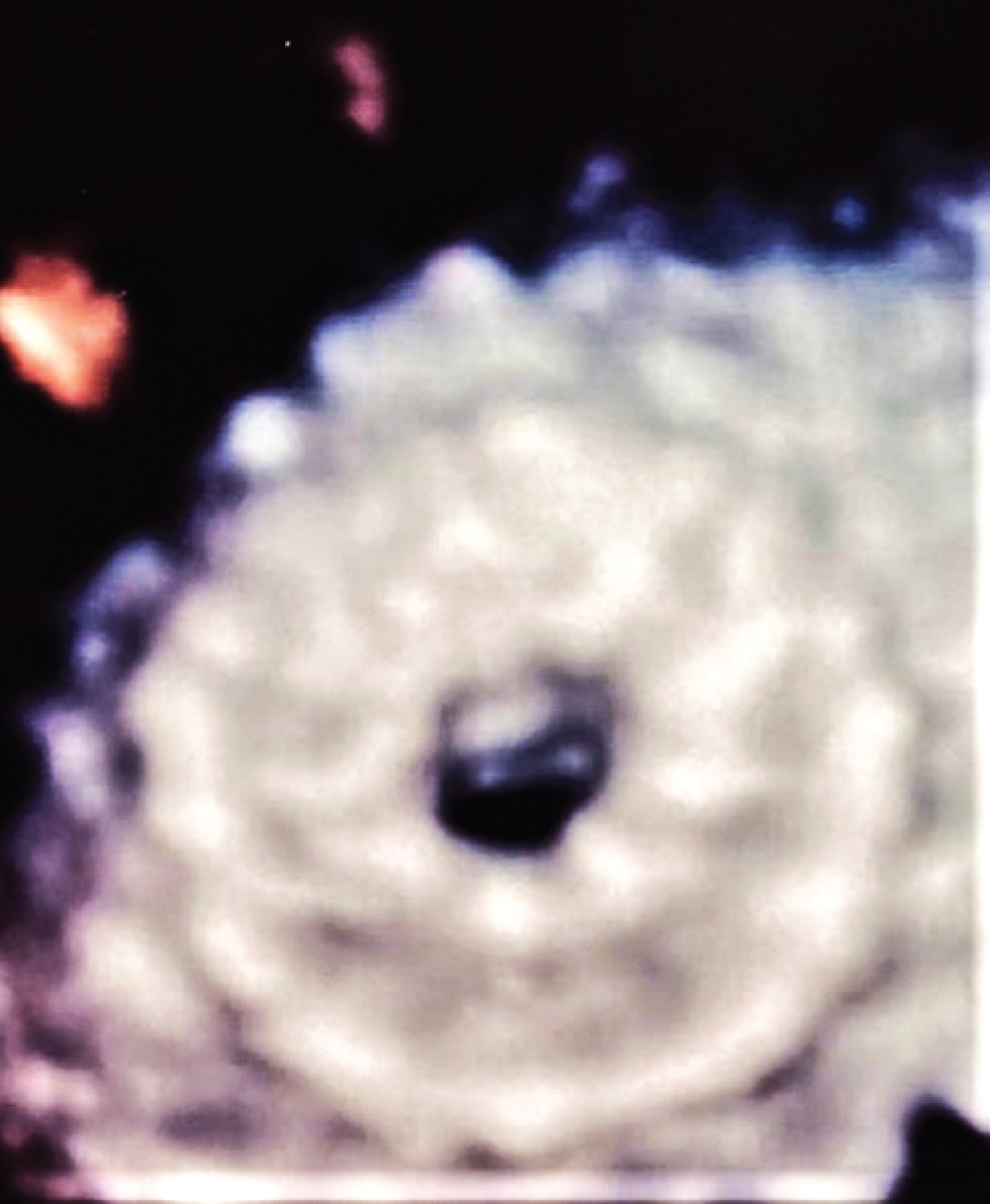 Figure 3: Zoomed 3D-Echocardiographic view of atrial flow regulator.
View Figure 3
Figure 3: Zoomed 3D-Echocardiographic view of atrial flow regulator.
View Figure 3
At the 6-month follow-up visit, the patient showed an improvement of his own clinical status. He switched from NYHA III to NYHA II functional class. At trans-thoracic echocardiography, there was a preserved left-to-right atrial shunt without any right heart overload (TAPSE 21 mm, PAPs 30 mmHg). Ultrasonographic LAP has been reduced from 26 to 16 mmHg.
HF is a progressive clinical syndrome characterized by reduced cardiac output and/or elevated cardiac pressures [1]. Patients with chronic HF usually present elevated LAP [8], especially at exercise, which leads to excessive rise in PCWP, and subsequently causes HF symptoms, like dyspnoea and reduced tolerance to physical activities [13,14]. Moreover, increased LAP might be the pathophysiological clue to justify the onset of decompensated symptoms in advanced HF. Experience in terms of IAST application in patients with HF is limited, but some scientific evidences indicate that the generation of an inter-atrial shunt is able to improve both clinics and haemodynamics in patients presenting elevated filling pressures. For example, the atrial septal defect (ASD) limits the risk of pulmonary congestion in patients affected by Lutenbacher syndrome with mitral stenosis and, more interestingly, these subjects do not suffer from right HF or an increased risk of stroke at follow-up [15]. On the other hand, early HF onset with acute pulmonary congestion and atrial volume overload was observed in adult patients following transcatheter closure of ASD [16]. Invasive measurement of LAP, guiding medical therapy in patients with HFrEF, was associated with improved symptoms and higher decompensation-free survival [17].
IAST has been proposed as a novel strategy to reduce LAP and PCWP in order to ameliorate symptoms and improve functional status among patients with advanced HF [10-12,18]. Different IASDs (inter-atrial shunt devices) have been investigated in clinical trials: the Corvia Medical device (Corvia Medical Inc., Tewkesbury, MA, USA) produced a significant reduction of PCWP in patients with HFpEF in a small nonrandomized trial [18,19]; the V-Wave device (V-Wave Medical, CA, USA) demonstrated initial safety and early beneficial clinical and hemodynamic outcomes in patients with HFrEF although the benefits were compromised by impaired shunt patency on long-term follow-up [20]; the AFR led to improvement of symptoms and surrogate parameters of HF (NYHA functional class, quality of life assessed with the Minnesota Living With Heart Failure Questionnaire, distance covered at 6MWT) early at 6 months after implantation [21]. Each device was associated with a very low risk of post-procedure side adverse events [22]. Table 1 summarises the main characteristics and the clinical impact of the different percutaneous IASDs [23].
Table 1: Overview of studies about atrial shunt in patients affected by heart failure. View Table 1
In IAST-based clinical trials, most HF patients developed a significant improvement in terms of symptoms (evaluated with NYHA classification), haemodynamic parameters (especially in terms of PCWP reduction) and quality of life (evaluated with scores like the Kansas City Cardiomyopathy Questionnaire) [18-22].
The case reported here is a kind of post-ischemic HF with effort dyspnoea resulting refractory to optimal medical therapy. Following adequate clinical evaluation, patient was judged as an optimal candidate to IAST. In fact, he presented elevated PCWP at rest and no right heart overload. An 8-mm-diameter AFR was implanted without any complication. After the procedure, patient developed an important clinical benefit. At 6 months, there were a reduction of LAP and a 1-step-improvement of NYHA functional class.
So, despite it seems to have no prognostic impact, IAST appears a feasible and effective strategy to ameliorate quality of life in advanced HF. Further studies are necessary in order to better define long-term hemodynamic consequences of creating a percutaneous inter-atrial shunt. However, some evidence would highline the idea that IASDs are not noxious to cardiac physiology; in fact, it has been shown that smaller ASDs in adults (diameter < 10 mm and Qp:Qs ratio < 1.5) do not cause right ventricular volume overload, pulmonary hypertension, or right HF [24].
However, whether this interventional treatment is an effective therapeutic option on top of optimal standard medical care for HF patients with elevated filling pressures needs to be proved in randomized phase III trials.
The management of drug-refractory HF is challenging. Elevated LAP and PCWP are typical of this condition and seem to be associated with a worse clinical status. Inter-atrial shunt therapy through the implantation of an AFR device (Occlutech™) has been showed to be a safe and effective approach in reducing PCWP, ameliorating symptoms and improving functional class in patients affected by refractory HF.
The authors have no conflicts of interest to declare.
The authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.