Introduction: Myocarditis is a disease that has varying degrees of clinical manifestations. Furthermore, its diagnosis and management can pose as a challenge to clinicians. With over 250 million people receiving the mRNA covid vaccine, there have been rare reports of myocarditis, pericarditis or other cardiovascular involvement. However, its natural course remains unclear.
Case: We report a 30-year-old healthy male who was hospitalized 3 days after the second dose of the covid-19 vaccination. He had high cardiac laboratory markers (cardiac troponin I peak of 22 ng/mL, normal < 0.03 ng/mL). He had a subsequent coronary angiogram and echocardiogram that were normal and was hence diagnosed with myocarditis. After week 3, his symptoms fully abated along with all of his inflammatory and cardiac markers. He had a cardiac magnetic resonance (CMR) study 8 weeks later (from the date of his vaccine dose) that showed prominent mid to epicardial lateral wall fibrosis with normal T1 and T2 mapping. A repeat CMR was performed 3 months later, which is almost 22 weeks since the vaccine administration. It showed persistent, though partially resolving fibrosis, despite full resolution of symptoms and biomarkers, demonstrating an insidious recovery.
Conclusion: This case demonstrates myocarditis following the second dose of the mRNA Covid-19 vaccination with evidence of myocardial fibrosis on CMR and on follow-up CMR scans nearly 5 and ½ months from administration. Furthermore, it shows remaining evidence of myocardial fibrosis despite the normalization of symptoms and traditional screening methods such as echocardiography and the use of cardiac and inflammatory biomarkers in disease surveillance. It provides insight into its natural course and insidious recovery as well as the utility of CMR in clinical management.
Cardiac magnetic resonance imaging, CMR, Fibrosis, Covid vaccine, Myocarditis
CMR: Cardiac Magnetic Resonance; COVID-19: Coronavirus Disease 2019; VAERS: Vaccine Adverse Event Reporting System; Tmax: Maximum Recorded Temperature; FEU: Fibrinogen Equivalent Unit; WBC: White Blood Cells; ESR: Erythrocyte Sedimentation Rate; CRP: C-Reactive Protein; ECG: Electrocardiogram; LGE: Late Gadolinium Enhancement
Myocarditis is a heterogeneous disease associated with acute or chronic inflammation of the myocardium with extremely varied clinical manifestations, ranging from mild to severe [1-5]. The association between myocarditis and other cardiovascular involvement from the covid-19 vaccines (Pfizer-BioNTech and Moderna) was notified by the US Centers for Disease Control and Prevention's safety committee on the June 23rd, 2021 [6]. As more than 250 million people have received at least one dose of COVID-19 vaccine in the United States, as of February 5th, 2022; rare reports of myocarditis and pericarditis have been made to the Vaccine Adverse Event Reporting System (VAERS) (I.e: Pfizer-BioNTech, Moderna) in the United States [7-10].
We report a case of a young adult who presented to our hospital with acute myocarditis 3 days after receiving the second dose of the mRNA COVID-19 Vaccination. Cardiovascular Magnetic Resonance Imaging (CMR) was performed seven and half weeks later that showed myocardial fibrosis of the lateral wall. A follow-up CMR (3 months later, near month 6 after vaccine administration) showed persistent fibrosis, although diminished, despite the full resolution of symptoms and normalization of inflammatory and cardiac biomarkers.
A 30-year-old male with no significant past medical history presented to our emergency department with substernal chest pain, 3 days after he had received the second dose of the mRNA COVID-19 Vaccine. He experienced flu-like symptoms including subjective fevers (reported maximum recorded temperature or Tmax < 100 degrees Fahrenheit) and general malaise shortly after vaccine administration. He woke up at 5 a.m. with a sensation of pressure like chest pain that was substernal and associated with diaphoresis and mild nausea that prompted emergency room visit. His prior dose of the vaccine was several weeks earlier, and he reported a mild flu-like illness that was self-limiting. He denied shortness of breath, cough, rhinorrhea, anosmia, or recent sick contacts. There was no significant social history or a history of illicit drugs or alcohol use.
Initial labs revealed a significant elevation in Troponin to 15.4 ng/mL (normal range < 0.03 ng/mL). D-dimer was < 0.27 ug/ml Fibrinogen Equivalent Unit (FEU), normal range < 0.50 ug/mL FEU). A computed tomography angiography of his pulmonary arteries with intravenous contrast was performed that showed no evidence of pulmonary emboli, aortopathy or acute pulmonary pathology but revealed lymphadenopathy; which was presumed to be secondary to inflammatory mediated. White blood cells (WBC) were 7 (thousand per cubic milliliter) K/uL (normal range: 4.5-10.3 K/uL). Erythrocyte sedimentation rate (ESR) was 11 (millimeters per hour) mm/hr (normal range 0-15 mm/hr). C-reactive protein (CRP) was 40.86 (milligrams per liter) mg/L (normal range: < 5.1 mg/L). Repeat Troponin trended upwards and peaked at 22.3 ng/ml. Initial electrocardiogram (ECG) is listed in Figure 1. A repeat ECG revealed ST segment elevation in the inferior-lateral leads, Figure 2. Chest X-ray was without significant findings.
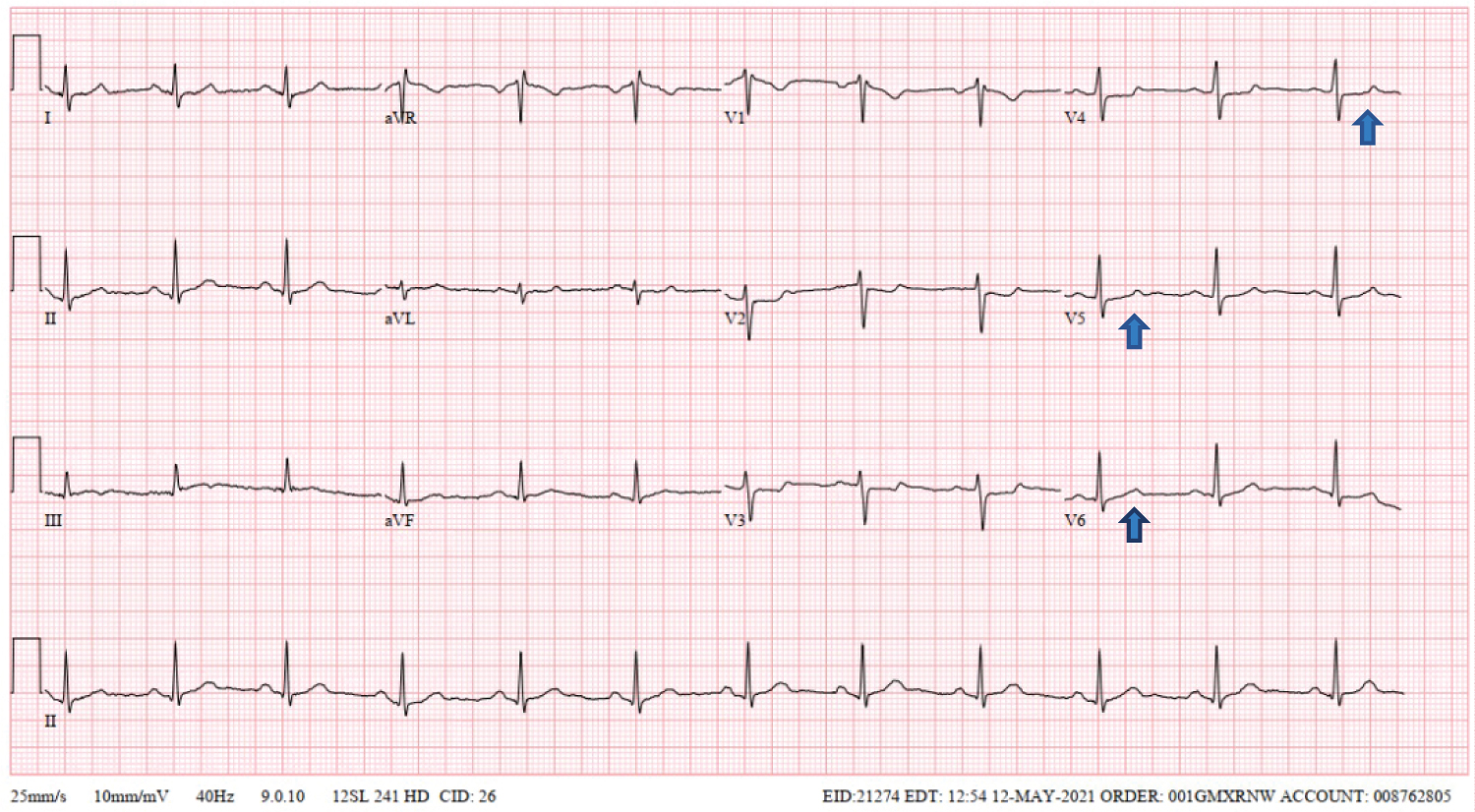 Figure 1: First ECG on the day of admission- showing sinus rhythm with T-wave inversions and ST segment depressions in precordial leads V2-V4 (arrows).
View Figure 1
Figure 1: First ECG on the day of admission- showing sinus rhythm with T-wave inversions and ST segment depressions in precordial leads V2-V4 (arrows).
View Figure 1
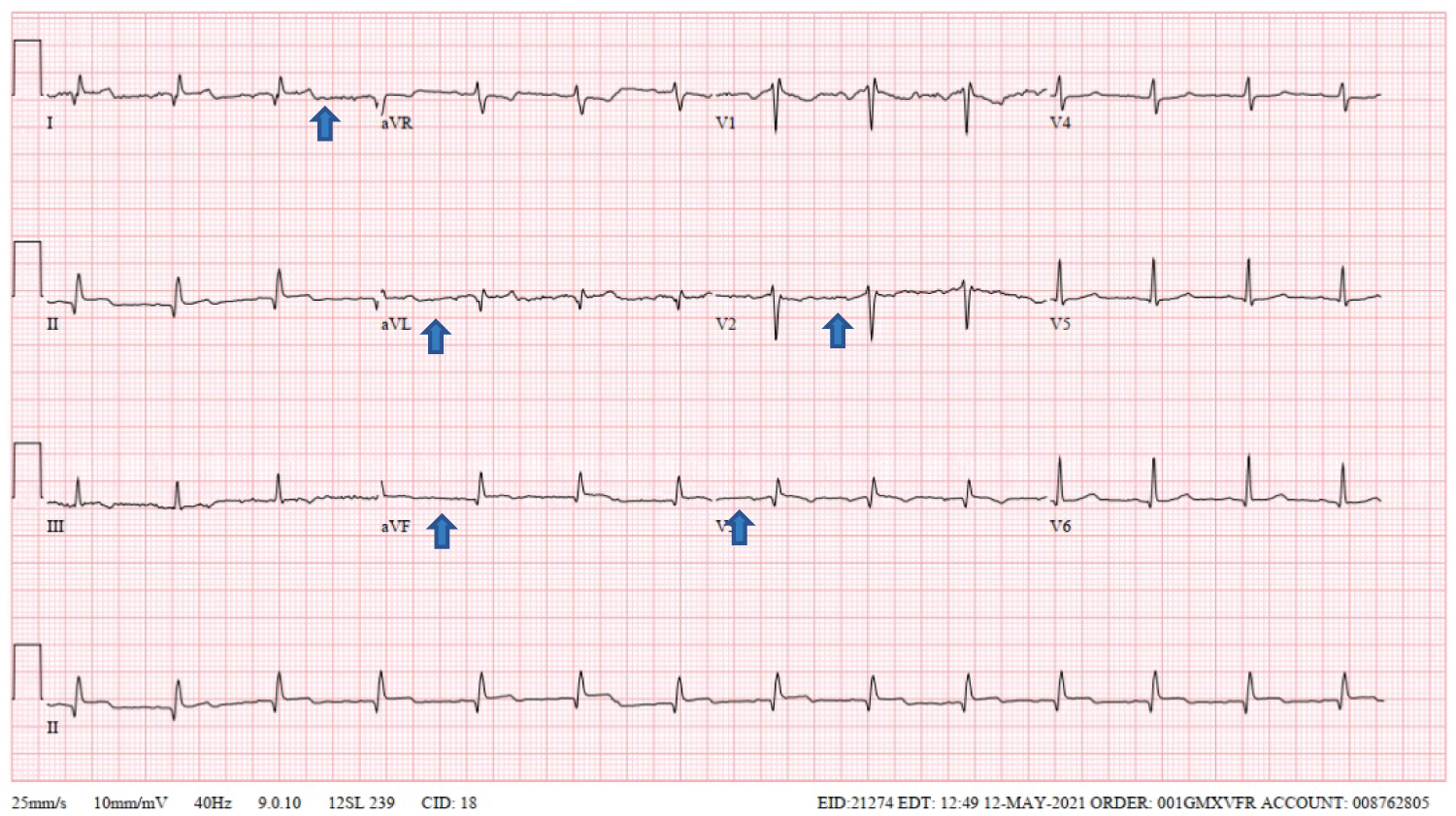 Figure 2: 2nd ECG on day 2- Sinus rhythm with nonspecific ST and T wave abnormality lead V3, and infero-lateral ST segment elevations (arrows) with low voltage.
View Figure 2
Figure 2: 2nd ECG on day 2- Sinus rhythm with nonspecific ST and T wave abnormality lead V3, and infero-lateral ST segment elevations (arrows) with low voltage.
View Figure 2
The patient was started on aspirin, atorvastatin, unfractionated heparin and ticagrelor. He was initially treated as an acute coronary syndrome due to the nature of his pain, cardiac enzymes, and ECG abnormalities. On the second day of hospitalization, the patient had undergone a coronary angiogram, which revealed no coronary artery disease. Transthoracic echocardiogram revealed no regional wall abnormalities and a left ventricular ejection fraction (EF) > 55% with a normal right ventricular ejection fraction. The patient was started on colchicine and ibuprofen as needed for the clinical diagnosis of myo-pericarditis. The patient reported subsequent resolution of all symptoms 3 weeks later. Seven and a half weeks (since the second dose of COVID-19 Vaccine), the patient underwent CMR, using General Electric Signa Artist 1.5 Tesla with field of view 36 × 32 mm, slice thickness 8 mm, 0 mm spacing, matrix 200 × 200 pixels mm, number of excitations 1. Gadolinium-enhanced imaging was performed approximately 10 min after administration of 0.1 mmol/kg body weight of gadobutrol (Gadovist; Bayer). It revealed evidence of mid-myocardial fibrosis of the basal inferolateral and lateral walls. There was homogenous signal intensity on T2 weighted images with normal native T1/T2 values on corresponding maps throughout the ventricle (Figure 3 and Figure 4 a-e). The pericardium was notably normal in thickness with no evidence of effusion or constriction. The patient underwent follow-up CMR 3 months later (from prior scan) to document resolution of his fibrosis, however, it showed persistent, though mildly diminished, fibrotic area in the same corresponding territory as prior scan and normal T1 and T2 values on respective T1/T2 maps. The patient remained symptom free.
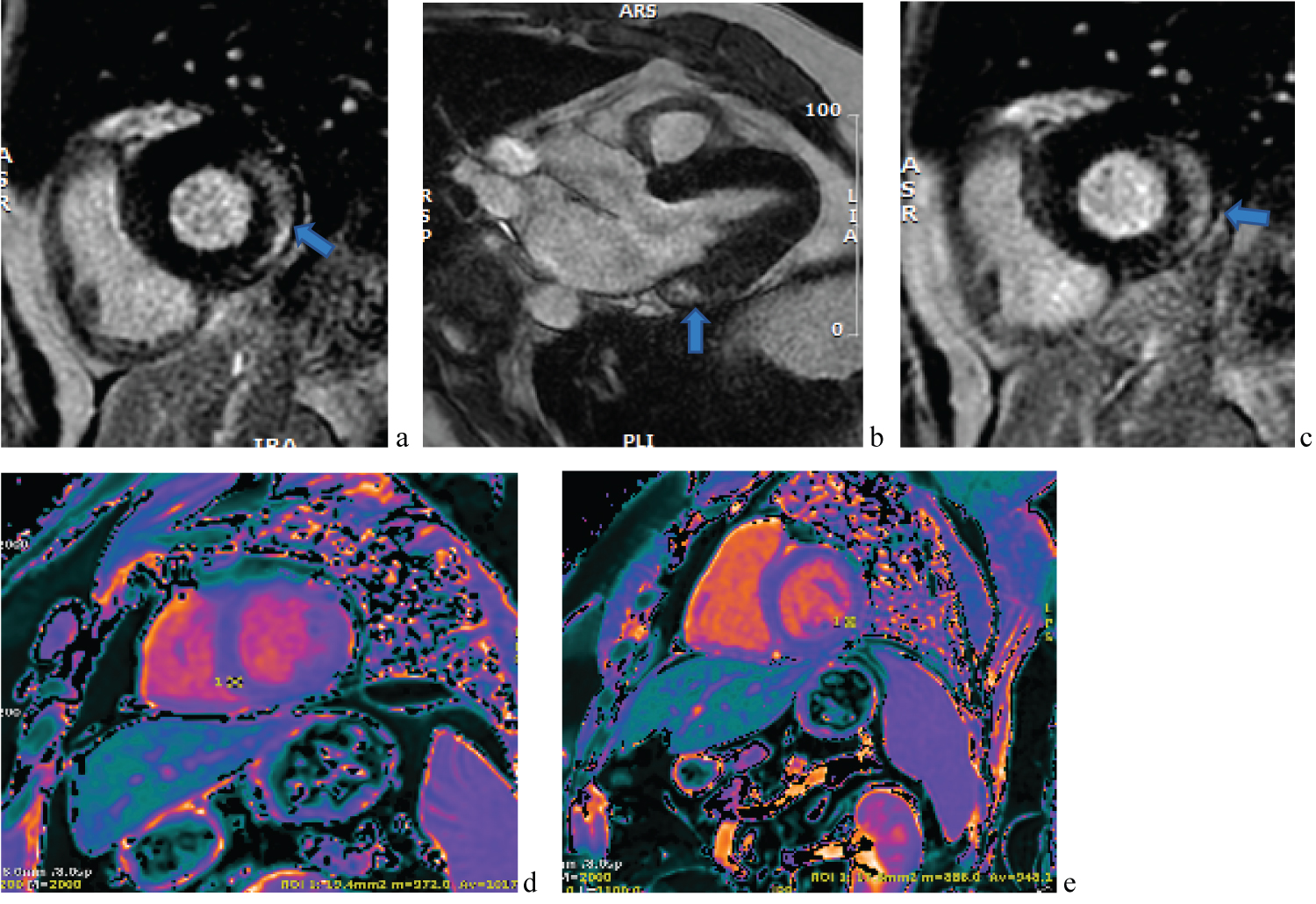 Figure 3: (a-e) First CMR scan with mid to epicardial lateral wall fibrosis (in white) with LGE (arrows) on gradient echo images and T1 maps (normal).
View Figure 3
Figure 3: (a-e) First CMR scan with mid to epicardial lateral wall fibrosis (in white) with LGE (arrows) on gradient echo images and T1 maps (normal).
View Figure 3
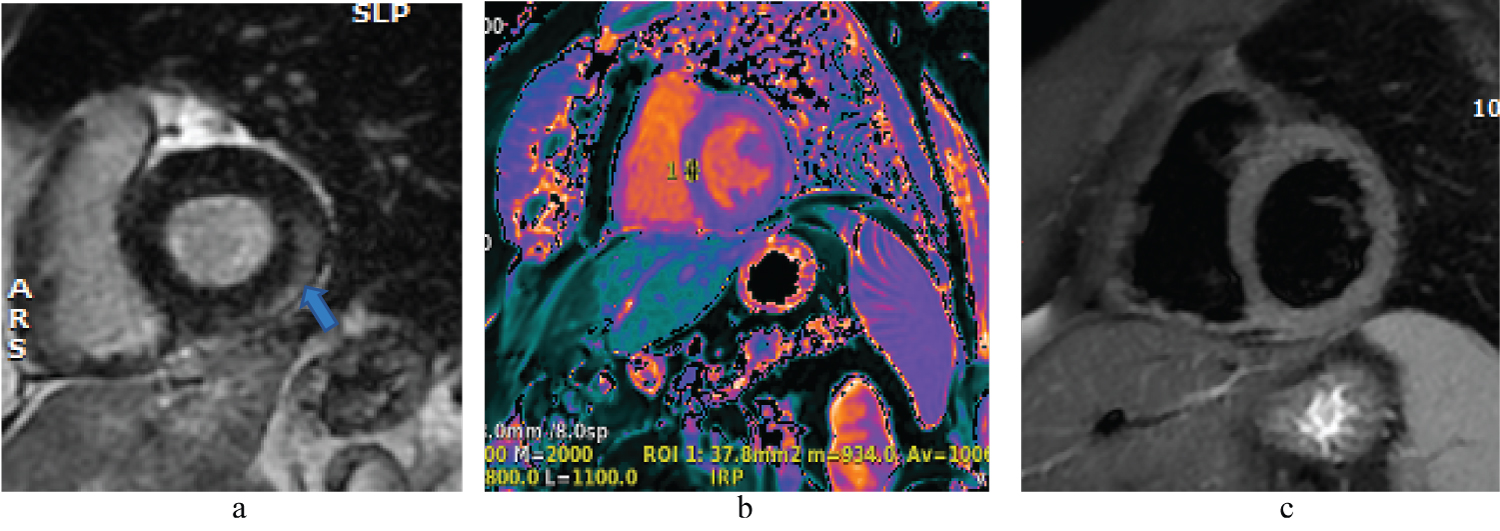 Figure 4: (a-c) Follow-up CMR with partial resolution of lateral fibrosis (arrow). a) LGE on gradient echo; b) is T1 map with uniform signal; c) T2 weighted image uniform without myocardial edema.
View Figure 4
Figure 4: (a-c) Follow-up CMR with partial resolution of lateral fibrosis (arrow). a) LGE on gradient echo; b) is T1 map with uniform signal; c) T2 weighted image uniform without myocardial edema.
View Figure 4
Recently published reports suggest the rare occurrence of myocarditis in the young males, with less than 106 cases per million doses of the COVID-19 vaccine and in most instances within the first week after receiving the second dose of the COVID-19 vaccine [6]. Almost one hundred percent of them had significantly elevated cardiac troponin levels (10-fold to 400-fold the upper limits of their respective reference ranges) [2,7,10]. Other recent reports suggested that prior exposure to either the COVID-19 vaccine (1st dose) or COVID-19 infection prior to the 1st dose of vaccine significantly increased the risk of myocarditis after the second exposure (to the first dose after COVID-19 infection) or after the second vaccine (considering the first dose as the first exposure) [5,11].
Due to the wide variation in symptoms and or severity of illness in myocarditis, ranging from self-limiting to fulminant heart failure with cardiogenic shock and or death, the diagnosis and surveillance of myocarditis can be a challenge to clinicians [12-15]. Often it's diagnosis is made clinically based on various exposures to viral, infectious or immunization exposures [14]. Laboratory makers such as high sensitivity troponin assays as well as the evaluation of cardiac function via echocardiography are often the primary methods for its diagnosis, while myocardial biopsy is reserved for life threatening cases and is considered the gold standard. Without biopsy, there is not one widespread sole method for diagnosing myocarditis and often the laboratory, imaging and clinical histories together are used. Furthermore, despite the high prevalence of elevated laboratory biomarkers in acute phases, the utility in using such markers to aid in assessment of resolution or chronic phases is not well studied, and hence, the interval monitoring of progression or resolution of disease can be equally challenging.
CMR is the gold standard in detecting cardiac anatomy and ventricular function and has shown to have high accuracy for the diagnosis and surveillance of myocarditis [16,17]. Patients with acute myocarditis have been found to have significantly lower ejection fraction and wall motion abnormalities in comparison to echocardiography [18]. The tissue based CMR markers, such as T2-weighted ratio, early gadolinium enhancement, and late gadolinium enhancement (LGE) are used by The Lake Louise criteria, which is currently the recommended diagnostic criteria [19,20]. LGE effectively identifies the areas of damage of myocardium with high affinity [9]. The damage in the myocardium can be visualized after intravenous injection of gadolinium, which is retained for prolonged period in the damaged areas, such as scarred, fibrosed or edematous areas of the myocardium, due to different wash-in and wash-out kinetics [4,9]. This test has a reported sensitivity of 100% and specificity of 90% [4,9].
Further advances in CMR have used T1 and T2 mapping with or without extracellular volume assessment and have shown to augment the diagnostic utility of CMR in myocarditis [16,19,21,22]. Myocardial ischemia, infarction or edema in focal areas of the myocardium can be detected by T1 or T2 weighted sequences with mapping [16]. T1 maps can detect edema in infarction with a high sensitivity and specificity; and can detect lipomatous metaplastic changes in chronic myocardial infarction where myocardial cells are fibrosed, and eventually replaced by extracellular collagen tissue [16]. On the other hand, T2- weighted sequences can assess myocardial edema in ischemic and non-ischemic heart diseases, such as myocarditis, Takotsubo cardiomyopathy, transplant rejection [16,23]. Some studies suggest that myocardial abnormalities can be detected to a greater extent along with the differentiation of the convalescent stages from the acute stage by the native T1 mapping when compared to T2-weighted imaging and LGE [21]. Whereas other studies suggest that acute myocarditis can be differentiated from recent heart failure by myocardial T2 values [21]. A summary of imaging techniques to compliment myocardial assessment for myocarditis is shown in Figure 5, borrowed from Karamitos ED, et al. [24].
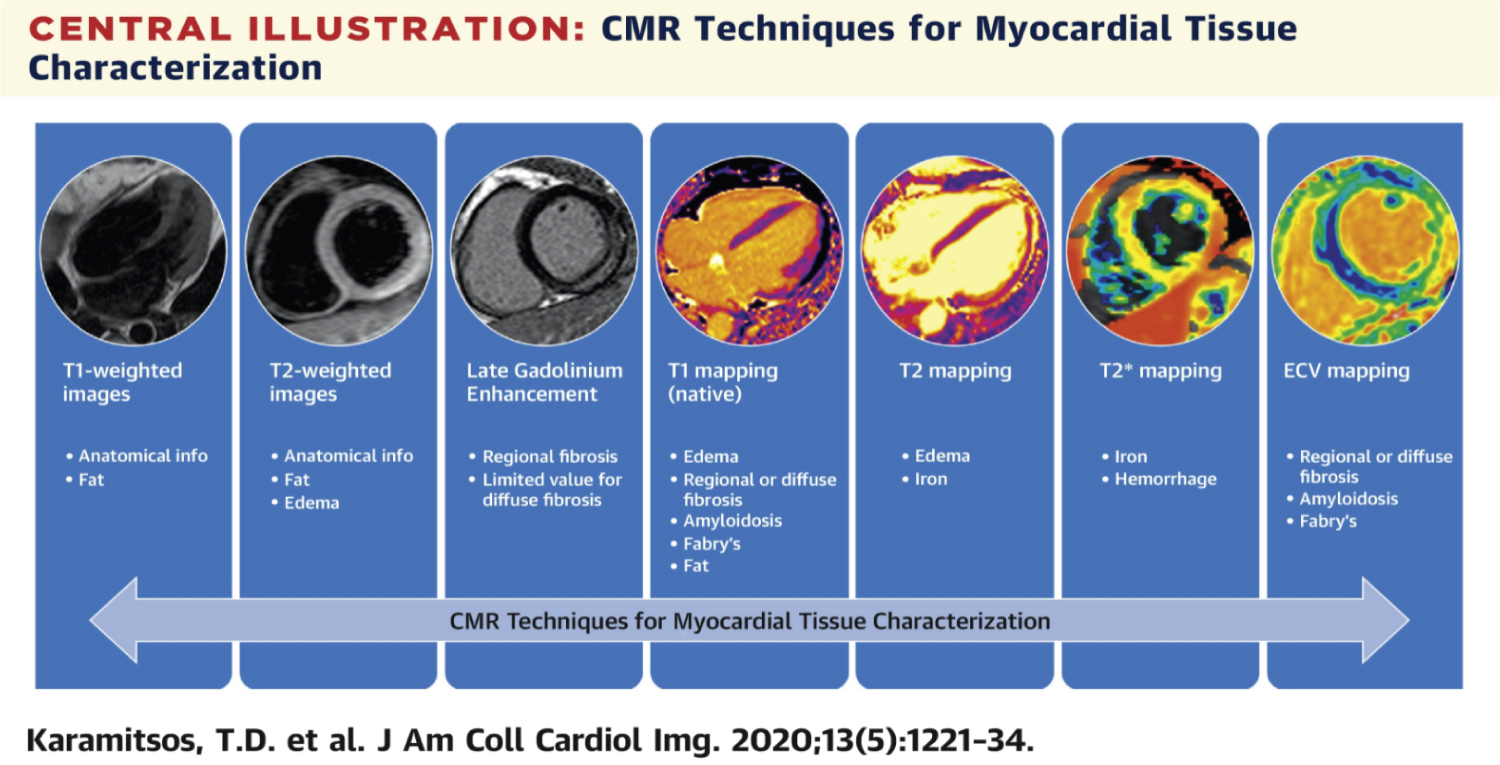 Figure 5: Borrowed from Karamitsos TD, et al. illustrating various CMR imaging techniques used in quantification of myocardial fibrosis and edema.
View Figure 5
Figure 5: Borrowed from Karamitsos TD, et al. illustrating various CMR imaging techniques used in quantification of myocardial fibrosis and edema.
View Figure 5
Our findings demonstrate multiple parameters that confirm myocarditis but also suggest a dynamic process demonstrating an insidious course and slow resolution. Our patient had significant ECG changes along with marked elevated cardiac biomarkers without evidence of coronary artery disease and in conjunction with recent vaccine exposure and elevated inflammatory markers (CRP). He was hence diagnosed and treated for myo-pericarditis. Despite high cardiac markers, he had normal left ventricular function on echocardiography without wall motion. His CMR was done 7 and a half weeks after his second vaccination and showed prominent LGE of the lateral wall. It is noteworthy that there have been studies to suggest that viral myocarditis has a higher predominance of involving the lateral wall [17,18]. It remains unclear to us if this similarity following the vaccine administration is coincidental or related to mRNA technology. It is also of note that at the time of first CMR scan, the patient was symptom free and there was no concurrent myocardial edema on T2 weighted imaging or abnormalities on T1 or T2 mapping, respectively at the time if his first scan. Due to the normal T1, T2 weighted images, as well as T1 and T1 mapping, we, hence, suspected that his myocarditis was subacute or resolving. We, hence, recommended close follow-up, holter monitoring for arrhythmias and limitation of intense aerobic activities until further resolution of his fibrosis was demonstrated. We chose to repeat CMR scan in 3 months due to his lack of symptoms, however, this strategy was also not well defined in any current guidelines. To our surprise, his repeat CMR showed a persistent scar, though mildly diminished, despite remaining symptom free and with persistent normalization of all cardiac and inflammatory biomarkers. Due to the resolution of his clinical symptoms, we did not feel that myocardial biopsy or immunomodulation or systemic steroids were indicated. To the best of our knowledge, this is the only CMR based study that has documented resolving fibrosis in the setting of vaccine related myocarditis, despite normalization of all symptoms and biomarkers that we were able to monitor. Overall, it correlates with a benign clinical course and sheds information on an insidious and slow resolution of myocardial scar that may persist and lag in the time after improvement of symptoms and other biomarkers. Further and larger studies are suggested.
This case demonstrates persistent myocardial fibrosis via CMR 2 and near 6 months from illness after the second dose of the mRNA vaccine. Although persistent, the fibrosis has mildly diminished despite the normalization of all inflammatory markers, cardiac biomarkers and symptoms. It provides insight into its natural course with an insidious recovery as well as the utility of CMR in aiding clinical management.
• Ethical approval was not applicable for this paper.
• Consent for publication was obtained. All images and data have been kept anonymous.
• Availability of data and materials is available upon request.
• All authors report no competing interests, funding or financial disclosures.
• AFM Ashik Imran is a primary author.
• Won Jun Park is a contributor and editor.
• Michael Sood is an additional primary author, editor and mentor.
We would like to acknowledge the division of cardiology and department of radiology at Mount Sinai South Nassau, NY, USA.