Aim: Carotid-femoral pulse wave velocity (cf-PWV) is the gold standard measure of arterial stiffness and a stronger predictor of poor outcomes for cardiovascular events. Pulse wave velocity is closely related to blood pressure, but its effect on office hypertension (OHT) is unclear. The study aims to investigate the relationship between OHT and cf-PWV.
Materials and methods: This was a prospective, single-center, clinical trial. A total of 102 subjects were included in the study: 48 patients with OHT and 54 subjects with normal blood pressure. Clinical risk factors and biochemical parameters were noted. Subjects underwent an assessment of cf-PWV by the validated tonometric system (SphygmoCor). The healthy group and the OHT group were compared.
Results: Demographic and clinical parameters did not differ between groups. However, cf-PWV values were higher in the OHT group compared to healthy subjects. (9.0 ± 1.7 m/s vs. 7.9 ± 1.8 m/s, p = 0.002). The cf-PWV was correlated with systolic blood pressure, (r = 0.310, p = 0.002), and found as an independent predictor of OHT. ROC analysis identified that PWV levels had moderate sensitivity and specificity for predicting OHT.
Conclusion: The patients with OHT have higher cf-PWV values compared to healthy subjects. Pulse wave velocity plays a role in the pathophysiology of OHT, and is an independent predictor of it.
Office hypertension, Pulse wave velocity, Arterial stiffness
Studies have been demonstrated a better cardiovascular prognosis for isolated office hypertension (OHT) (white-coat hypertension) patients than those with sustained hypertension [1-4]. However, its prognostic significance is controversial when compared to healthy subjects [5,6]. Isolated OHT is associated with structural cardiac abnormalities, and increased left ventricular mass, a well-known cardiovascular risk marker, is one of these abnormalities [7-10].
Carotid-femoral pulse wave velocity (cf-PWV) is a measure of the intrinsic stiffness of the aortic wall and is a predictor of cardiovascular events. The prognostic importance of cf-PWV is that it is considered as an integral measure of the adverse hemodynamic effects of aortic stiffness [11]. Once aortic stiffness occurs systolic pressure and pulse pressure increase and myocardial perfusion pressure decreases [12-14].
High PWV values are involved in the pathophysiology of OHT. Hypertension leads to increased aortic stiffness and PWV, resulting in target organ damage. Office hypertension causes an increase in aortic stiffness and, therefore, an increase in PWV. So, PWV may be an independent predictor of OHT. In this study, we aimed to investigate whether PWV can be used to predict OHT.
The prospective study included 175 consecutive subjects, and after exclusion criteria were applied, 102 of them were analyzed. Blood pressure measurement at rest was performed with a sphygmomanometer, and at least two measurements above 140/90 mmHg were defined as hypertension [15]. Subjects were divided into two groups; the first group consisted of 48 hypertensive patients, and the second group consisted of 54 age and gender matched normotensive subjects. Subjects with sinus rhythm and normal left ventricular systolic function were included in the study. Patients with moderate/severe valvular disease, ascending aorta dilatation, coronary artery disease, peripheral artery disease, and carotid artery disease were excluded from the study.
Pulse wave velocity was calculated using SphygmoCor (AtCor Medical Instruments, New South Wales, Australia) branded tonometry device. First, suprasternal notch-femoral distance and suprasternal notch-carotid distance measurements were performed with a standard tape measure, and data were entered into the device. In the device, the "intersecting tangents" were used as the NDH algorithm, and it was set to compare the 10-second recordings. The pressure waveforms of the carotid and femoral arteries of the patients were measured noninvasively by placing the pressure-sensitive transducer in the relevant places on the neck and groin. The device automatically calculated NDH values by dividing the suprasternal notch-femoral distance and the suprasternal notch-carotid distance difference by the pulse wave transit time. Patients were excluded if the standard deviation was greater than 10%, or the difference between the pulses at the two measuring sites was more than five beats/min.
The SPSS statistical software (SPSS, version15.0; Inc., Chicago, IL, USA) was used for all statistical calculations. Continuous variables were given as mean ± SD or median [interquartile range] as appropriate; categorical variables were defined as percentages. Data were tested for normal distribution using the Kolmogorov-Smirnov test. The Student's t-test was used for the univariate analysis of normally distributed continuous numerical variables, and Mann-Whitney U-test was used for non-normally distributed numerical variables. Categorical variables were compared with the Pearson chi-square or Fisher exact test. Correlations between variables were tested using the Pearson correlation test for normally distributed variables and the Spearman correlation test for non-normally distributed variables. Variables with a p-value < 0.05 were included in the multivariate logistic regression analyses with the enter method. Receiver-operating characteristic (ROC) curve analysis was performed by using Med Calc software for variables that remained significant after multivariate analysis to. All tests of significance were two-tailed. All tests of significance were two-tailed. Statistical significance was defined as p < 0.05.
Office hypertension was detected in 48 of 102 subjects. The mean age of the patients was 52 ± 8 in the OHT group and 50 ± 10 in the healthy group (p = 0.06); male gender ratio was 42.1% (n = 20) in the OHT group and 33.1% (n = 18) in the healthy group (p = 0.4). There was no difference between the groups in terms of other characteristics (Table 1).
Table 1: Baseline characteristics of the study population. View Table 1
However, cf-PWV measurements of the OHT group were significantly higher than the control group (9.0 ± 1.7 m/s vs. 7.9 ± 1.8 m/s, p = 0.002) (Figure 1). The cf-PWV positively correlated with systolic blood pressure (r = 0.310, p = 0.002), age (r = 0.335, p = 0.001), and body-mass index (BMI) (r = 0.330, p = 0.001) (Table 2); and found to be an independent predictor of OHT (Table 3).
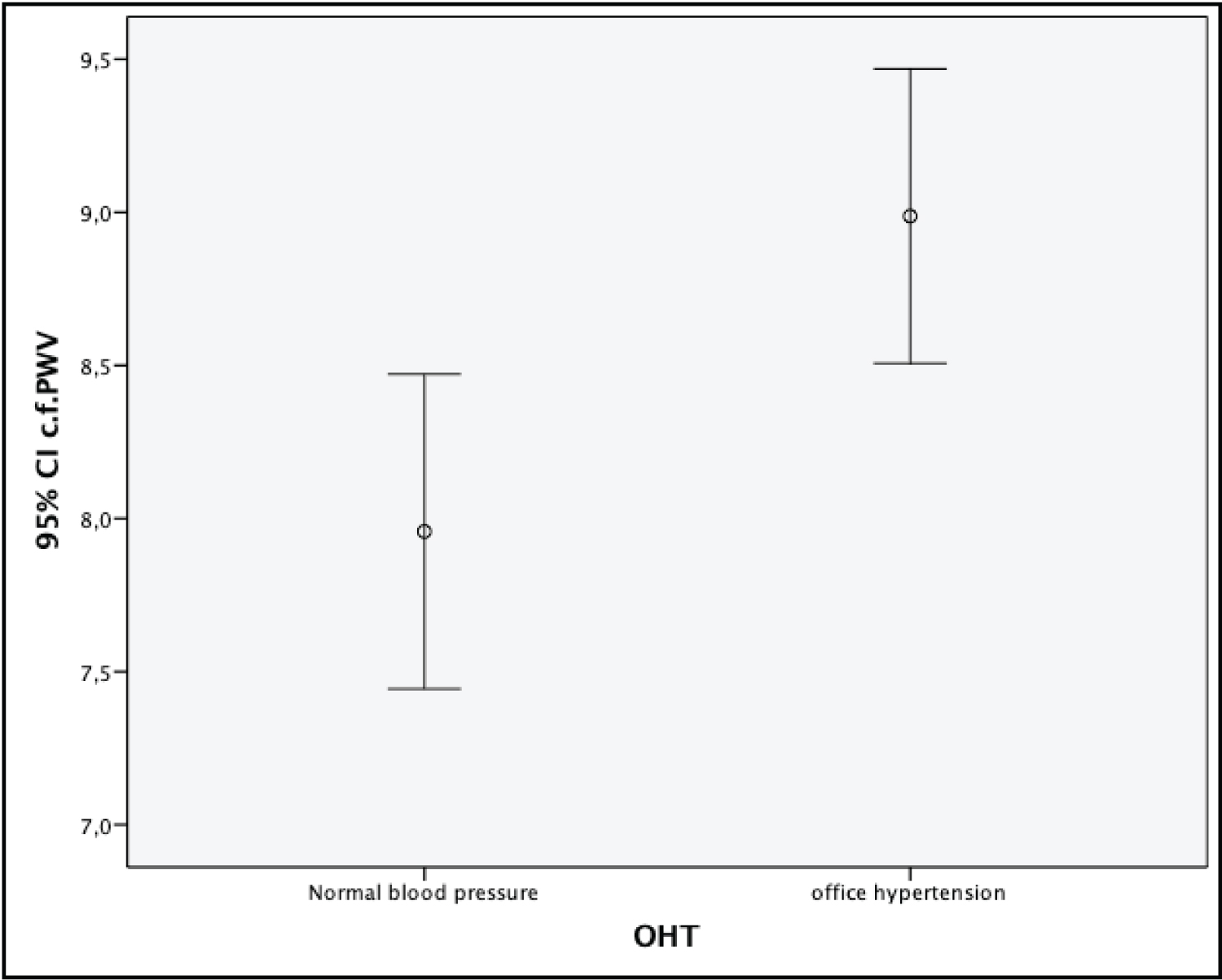 Figure 1: Association between aortic stiffness and wave reflection (cf-PWV) in patients with office hypertension (OHT) and normal blood pressure.
View Figure 1
Figure 1: Association between aortic stiffness and wave reflection (cf-PWV) in patients with office hypertension (OHT) and normal blood pressure.
View Figure 1
Table 2: Correlations of the surrogate markers with the parameters of Carotid-femoral pulse wave velocity (cf-PWV). View Table 2
Table 3: Results of multivariate analysis with logistic regression for OHT. View Table 3
We constructed the ROC curve to evaluate whether or not cf-PWV levels could be used as a screening tool to exclude OHT. The cf-PWV levels were likely to exclude OHT with a sensitivity of 69% and a specificity of 61% (area under the curve = 0.681, p = 0.002) using a cut-off value of 8.25 cf-pulse wave velocity (m/s) (Figure 2).
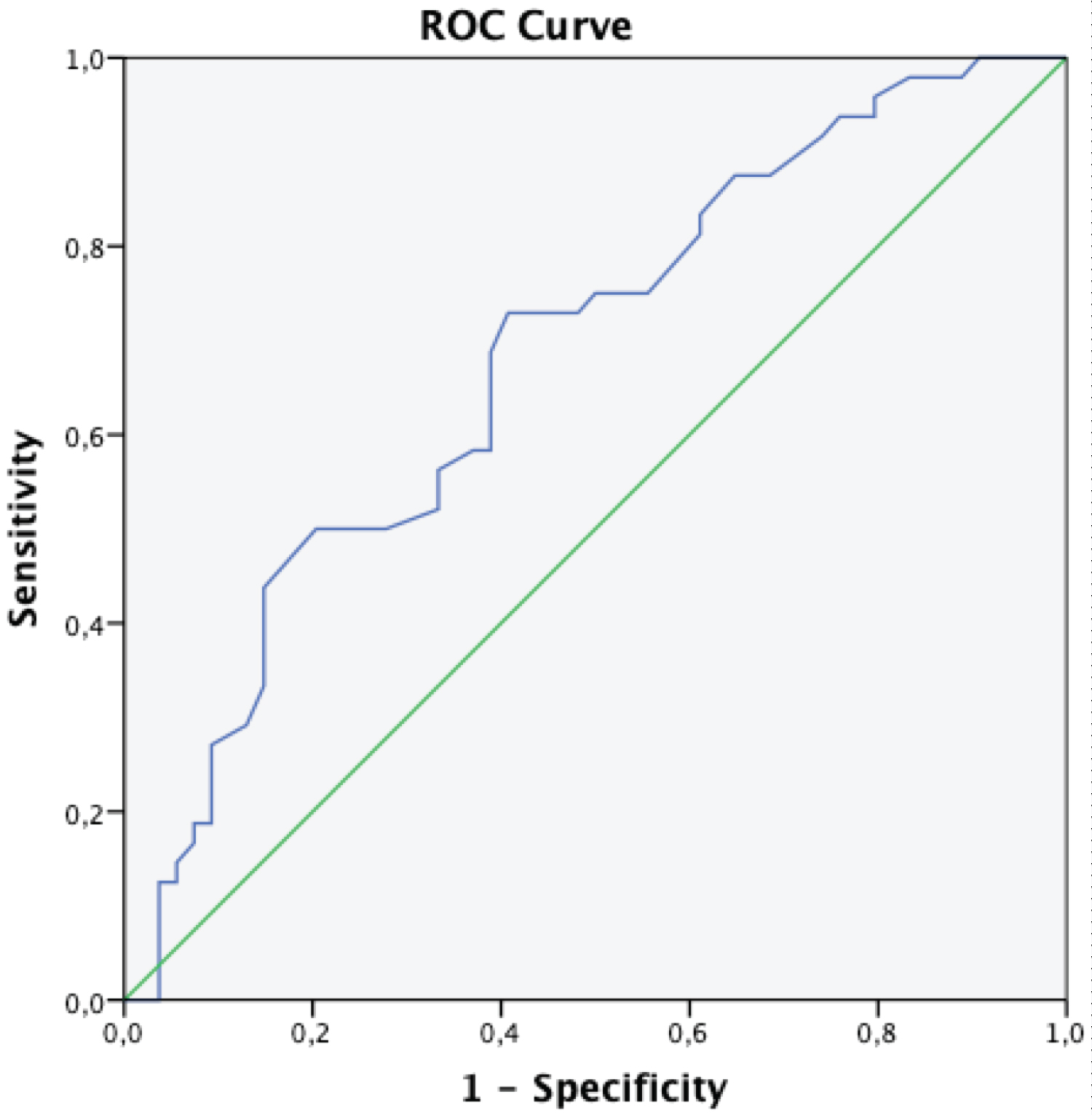 Figure 2: Receiver-operating characteristic curves (ROC) demonstrating the plot between sensitivity and specificity for cf-PWV levels for the diagnosis of office hypertension (OHT).
View Figure 2
Figure 2: Receiver-operating characteristic curves (ROC) demonstrating the plot between sensitivity and specificity for cf-PWV levels for the diagnosis of office hypertension (OHT).
View Figure 2
The current study evaluated the association between aortic stiffness and OHT, two markers of preclinical cardiovascular diseases. Study showed that subjects with isolated OHT had higher aortic stiffness and left ventricular hypertrophy prevalence than those with standard office/ambulatory blood pressure levels.
The systematic nature of the review provided the avoidance of bias in the choice of the study. Our findings confirm the well-established association of cf-PWV with age and blood pressure. Only a few studies have failed to show such relationships [16,17]. Their small sample size or narrow spread of age might cause it.
The significance of isolated OHT to predict future cardiovascular morbidity and mortality remains unsettled. Studies focusing on target-organ damage suggested that patients with isolated OHT might be at an intermediate risk between normotensive and those with sustained hypertension [18-20].
We may speculate that the increased left ventricular mass and aortic stiffness associated with uncontrolled isolated OHT may be due to neurohumoral, metabolic, or other factors irrelevant to blood pressure levels. An abnormal sympathetic response to environmental stimuli contributing to sudden rises in blood pressure can lead to cardiovascular damage [21]. However, the inadequacy of risk factors at the early stages of atherosclerosis on the stiffness of the arterial wall can explain the issue. Nevertheless, advanced plaque, particularly calcified, increases the stiffness of arteries [22]. This is supported by animal studies showing that PWV decreases in the early stages of a cholesterol-rich diet and increases with the development of atherosclerotic plaques [23]. It is possible that, in advanced plaque, where plaque volume may relate to cardiovascular risk factors, the relation of PWV to risk factors may differ [24].
Cf-PWV diverges from classical risk factors other than blood pressure in terms of atherosclerosis and cardiovascular events. This suggests that, at least in initial stages, aortic stiffening is presented by an alternative pathology other than atherosclerosis in which blood pressure is one of the most significant factors.
Arterial stiffness depends on a mechanical stretch of the arterial wall and, hence, on blood pressure at the time of the measurement [25]. Stretch is thought to cause the stiffening of the wall by transferring the load to the members having a higher tensile strength (e.g., from elastin to collagen) in the wall. It is difficult to distinguish the effects of a sustained elevation of blood pressure (hypertension) from the level of blood pressure at the time of the study. Hypertension probably causes structural alterations within the wall by accelerating age-related changes such as a decrease in elastin-collagen ratio, a change in collagen type, and the formation of collagen cross-links from advanced glycation [26,27].
The present review cannot figure out whether cf-PWV is associated with hypertension or blood pressure at the time of the study. However, given the predictive power of cf-PWV for cardiovascular events over conventional measures of blood pressure (including ambulatory blood pressure monitoring), it is crucial to identify the factors responsible for increased stiffness. The cf-PWV probably relates more to the duration and severity of hypertension that cannot be captured by a spot measure of blood pressure at the time of the study. In this regard, cf-PWV can measure blood pressure "better" than the conventional office measurement.
High PWV values play a role in the pathophysiology of OHT. Elevated blood pressure leads to increased aortic stiffness and PWV, causing target organ damage. Office hypertension may also lead to increased aortic stiffness, and thus PWV. Pulse wave velocity may, therefore, be an independent predictor of OHT. In the present study, we demonstrated that PWV could be used to predict and detect the OHT.
The patients with OHT have higher cf-PWV values compared with healthy subjects, and high PWV values play a role in the pathophysiology of OHT. Pulse wave velocity (PWV) is an independent predictor of OHT.
None (There is no potential conflict of interest regarding the article or its submission).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
The authors declared that the article submitted was responsible for the scientific content of the article in the Copyright Transfer Form. These areas of responsibility include design, data collection, analysis and interpretation, content writing, preparation and scientific review, and the final version of the paper.