In patients with CTEPH who are not eligible for PTE, BPA may be a treatment option. It is important to diagnose and treat patients early since delays are associated with worse clinical outcomes. We present a case of CTEPH where early diagnosis and treatment resulted in normalization of PA pressures.
Chronic thromboembolic pulmonary hypertension (CTEPH) is characterized by chronic thromboembolism in the proximal pulmonary arteries and small-vessel disease in the pulmonary capillary system [1]. This results in progressive remodeling of the pulmonary vasculature, increased pulmonary vascular resistance (PVR), pulmonary hypertension, right ventricular remodeling and ultimately right ventricular failure [2]. Early diagnosis of CTEPH is associated with improved outcomes, however, this can be challenging since patients may be asymptomatic for many years or present with non-specific symptoms [3,4]. Pulmonary thrombo endarterectomy (PTE) is the current gold standard treatment for CTEPH butmay not be an option for up to one-third patients. For such patients, balloon pulmonary angioplasty (BPA) has emerged as an efficacious alternative to PEA [5-9]. Here, we report the case of a patient with CTEPH who was diagnosed early and treated successfully with BPA.
• To realize the importance of early diagnosis in CTEPH given the association with better outcomes.
• To highlight that early diagnosis of CTEPH can be challenging.
• To recognize BPA as a treatment option for patients not eligible for PTE.
A 35-year-old male with a history of hereditary spherocytosis with splenectomy fifteen years prior, presented to cardiology clinic for evaluation of patent foramen ovale (PFO), right ventricular enlargement and elevated right-sided pressures observed on echocardiogram. Two months prior, he experienced an acute ischemic stroke, and during the work-up for his CVA an echocardiogram revealed severe pulmonary hypertension with a right ventricular systolic pressure (RVSP) of 80 mmHg. His right ventricle (RV) was dilated, had reduced systolic function, and there was intraventricular septal flattening during systole and diastole consistent with pressure and volume overload. The study also revealed a patent PFO. Transesophageal echocardiography later confirmed the presence of a moderate-sized PFO, which was successfully closed percutaneously with a 25 mm Gore Cardioform device. Right heart catheterization (RHC) performed at the time of PFO closure showed mean pulmonary artery pressure (mPAP) of 39 mmHg, pulmonary capillary wedge pressure (PCWP) of 10 mmHg and pulmonary vascular resistance (PVR) of 3 Woods Units. A computed tomography pulmonary angiogram (CTPA) revealed possible isolated subsegmental right lower lobe pulmonary embolus (PE) and a ventilation-perfusion (VQ) scan showed small to moderate-sized mismatched perfusion defects in the right lower lobe superior segment and anterior segment right upper lobe.
He underwent a repeat RHC about 8 months later, which showed mean pulmonary (mPAP) of 36 mmHg, PCWP of 17 mmHg and PVR of 3 Woods Units. VQ scan at that time redemonstrated multiple perfusion defects in the right lung, including the superior segment of the right lower lobe. High-resolution computed tomography (CT) showed no evidence of acute or interstitial lung disease. The patient's cardiologist suspected a diagnosis of chronic thromboembolic pulmonary hypertension (CTEPH) and referred the patient to a pulmonary hypertension (PH) referral center. At the time of referral, the patient had minimal limitations with his exercise capabilities, consistent with World Health Organization (WHO) functional class II.
His initial work-up was remarkable for a normal natriuretic peptide (NT-pro-BNP) concentration of 73 pg/mL. Echocardiography showed mild PH with RVSP of 43 mmHg. LV function and morphology were preserved. CTPA showed a perfusion defect in the right lower lobe (Figure 1A). RHC showed mPAP of 31 mmHg, PCWP of 17 mmHg and PVR of 2.53 Woods Units (Table 1). An invasive pulmonary angiogram revealed chronic obstruction of the A8 branch of the right pulmonary artery with poor filling of distal branches (Figure 1B).
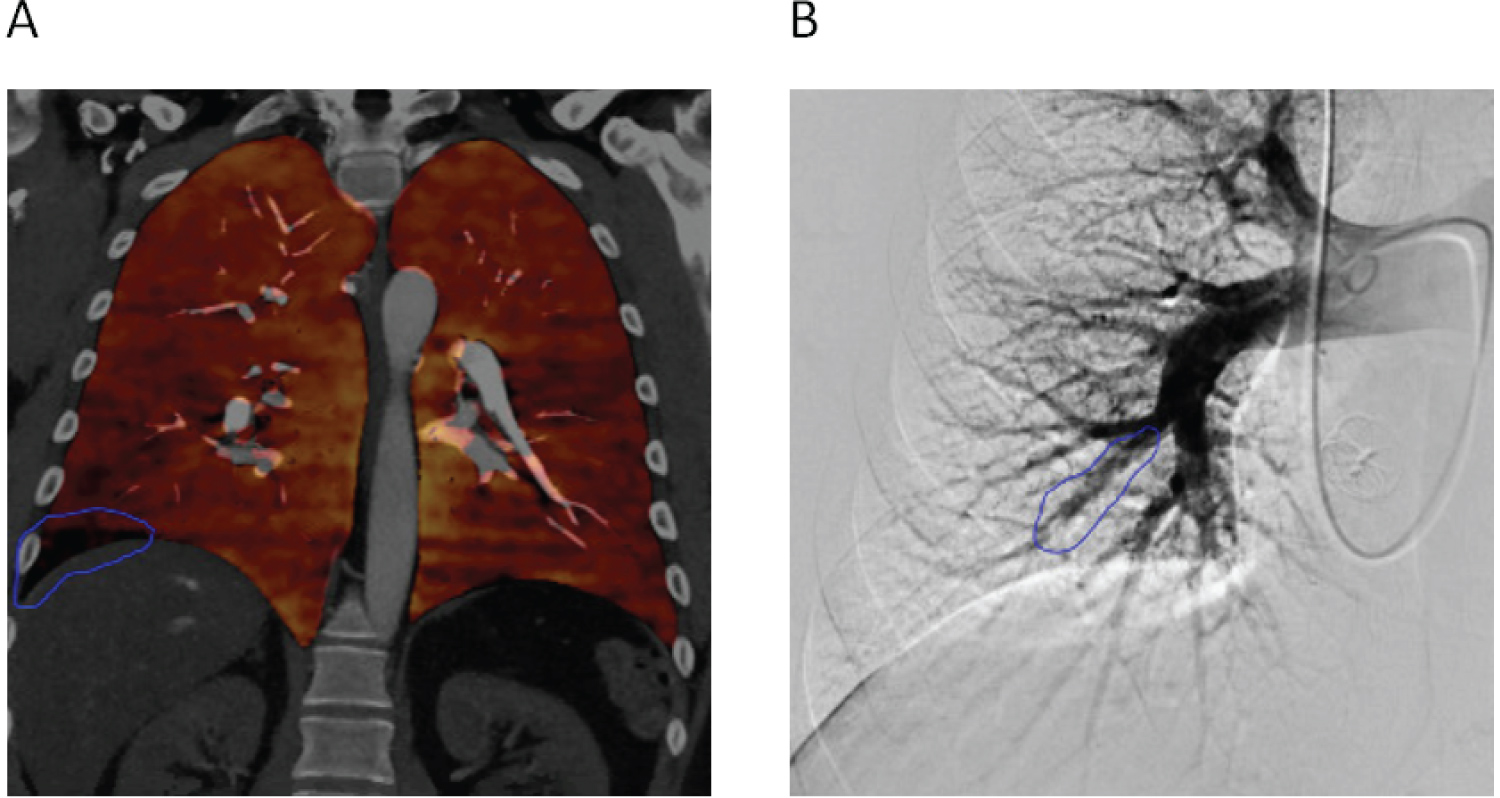 Figure 1: A) CT pulmonary angiogram showing perfusion defect in the right lower lobe (blue outline); B) Invasive pulmonary angiogram showing chronic obstruction of the A8 branch of the right pulmonary artery.
View Figure 1
Figure 1: A) CT pulmonary angiogram showing perfusion defect in the right lower lobe (blue outline); B) Invasive pulmonary angiogram showing chronic obstruction of the A8 branch of the right pulmonary artery.
View Figure 1
The patient was not a candidate for pulmonary thromboendarterectomy (PTE) since he had an isolated distal lesion. The decision was therefore made to perform a balloon pulmonary angioplasty (BPA) of the A8 branch of the right pulmonary artery. For the procedure, the patient's right femoral vein was accessed under ultrasound guidance without any difficulty. A long 8 French sheath was then placed the right pulmonary artery. Using this sheath, a 6 French JR4 guiding catheter was used to engage the right A10 segment and a weblike lesion in the proximal A10 segment was noted. A Sion blue wire was used to cross the A10 lesion and the lesion was serially dilated using 3.0 × 15 mm, 3.5 × 15 mm and 4.0 × 12 mm emerge balloons. Subsequently, a 6 French multipurpose guide was used to engage the right A8 segment. A Sion blue wire was used to cross the A8 lesion and the lesion was serially dilated using 3.0 × 15 mm, 3.5 × 15 mm and 4.0 × 12 mm emerge balloons with good results (Figure 2A, Figure 2B, Figure 2C and Figure 2D). There were no periprocedural complications.
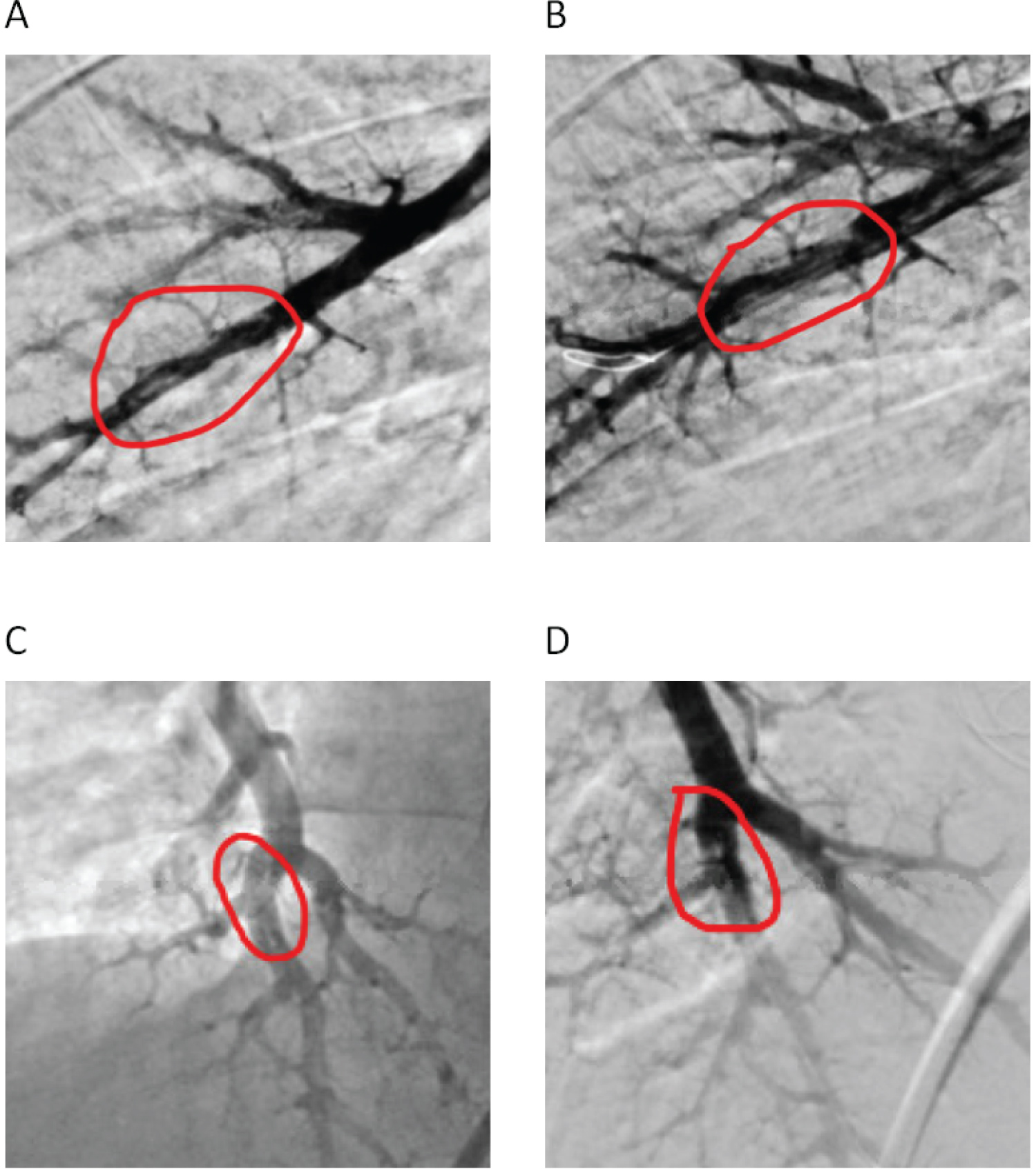 Figure 2: A) A8 segmental pulmonary artery pre-BPA and B) Post-BPA; C) A10 segmental pulmonary artery pre-BPA and D) Post-BPA.
View Figure 2
Figure 2: A) A8 segmental pulmonary artery pre-BPA and B) Post-BPA; C) A10 segmental pulmonary artery pre-BPA and D) Post-BPA.
View Figure 2
RHC performed one month after BPA showed mPAP of 21 mmHg (decreased from 31 mmHg), PCWP of 13 mmHg (decreased from 17 mmHg) and PVR of 1.16 Woods Units (decreased from 2.53 Woods Units) (Table 1). Echocardiography showed normal global RV and LV function. Patient is now WHO functional class 1.
Table 1: Values from right heart catheterization (RHC) before and after BPA. View Table 1
CTEPH, classified within group 4 PH, is characterized by chronic thromboembolism in proximal pulmonary arteries and small-vessel disease involving both the pulmonary venous and pulmonary arterial capillary systems [1]. These changes result in progressive remodeling of the pulmonary vasculature, increased PVR, pulmonary hypertension, RV remodeling and ultimately RV failure [2]. CTEPH should be considered in the evaluation of patients with pulmonary hypertension since it is treatable and potentially curable [8]. It is especially important to diagnosis and treat CTEPH earlier in the course of the disease, as delays in treatment have been associated with worse outcomes [4]. Making an early diagnosis of CTEPH, however, can be challenging since patients may be asymptomatic for many years or present with non-specific symptoms [3]. The presence of thromboemboli on CTPA and mismatched perfusion defects on VQ scan in a patient who has received at least 3 months of therapeutic anticoagulation is diagnostic of CTEPH [5].
PTE is the current gold standard treatment for CTEPH, however, about a third of patients may be deemed ineligible due to high-risk comorbidities, persistent pulmonary hypertension post-PTE or presence of chronic thromboembolism in the distal pulmonary arteries [6-9]. For such patients, BPA has emerged as an efficacious alternative to PTE, and has been reported to improve symptoms, hemodynamics, exercise capacity and right ventricular function when performed at an expert medical center [6,7]. In a meta-analysis by Kalra, et al. which included a total of 1604 patients (755 patients with inoperable CTEPH treated with BPA; 849 patients with inoperable CTEPH treated with pulmonary vasodilators) across 34 studies, BPA was associated with greater improvement in 6-minute walk distance, PVR and mPAP compared to pulmonary vasodilator therapy. BPA was notably associated with more complications due to its invasive nature [9].
As discussed above, CTEPH can be difficult to diagnose earlier in the course of the disease. The patient in this case was diagnosed at an early stage as part of the work-up for his CVA, which portends better outcomes. He had no prior history of PE, similar to 21.2% of patients with CTEPH in a study by Martinez, et al. who were found to have no history of PE [10]. Interestingly, this patient had a history of splenectomy, which is a known risk factor for CTEPH [1]. He was not a candidate for PTE since his initial evaluation showed an isolated distal lesion. He, instead, underwent BPA which normalized his PA pressures.