Interatrial block, Lateral amyotrophic sclerosis, Atrial fibrillation, Flecainide
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder affecting both upper and lower motor neurons. Cardiovascular consequences related to ALS are relatively under appreciated. Autonomic dysfunction in ALS is common. In fact, patients with ALS often exhibit bulbar dysfunction impacting centrally mediated vagal and sympathetic nerves with resultant greater autonomic dysfunction. The consequences of disruptions in the autonomic reflex loop on the cardiovascular system include chronotropic incompetence and vasomotor instability [1-3]. Arrhythmias associated with ALS may occur as a result of autonomic dysfunction. Bradycardia likewise can be explained in ALS by sympathetic denervation followed by the predominance of vagal function. Autonomic dysfunction begins with vagal release with enhanced sympathetic tone causing tachycardia. As patients later progress with ALS, sympathetic denervation occurs, followed by vagal predominance, leading to the development of bradycardia. In a study examining the effects of vagal tone on the sinoatrial node and AV node, in patients with a history of syncope, sinus arrest or bradycardia, it was found that increased vagal tone contributed to prolongation in the sinus cycle length [4]. The above may be the expression of the presence of denervation edema. In fact, in a previous study with cardiac magnetic resonance (CMR) was observed an early gadolinium enhancement which in a disease with denervation, as the main muscular pathology, is likely to be associated with denervation edema [5].
Interatrial block (IAB) is a frequent condition in old age, reaching 40% in the over 70+ year-old individuals [6,7]. Inter-atrial block is strongly associated with supraventricular arrhythmias, particularly with AF in many clinical contexts. Furthermore, IAB increases the risk of stroke [7] and appears to be associated with dementia.
The association of IAB with the incidence of AF was confirmed in different contexts [8-10]. We describe here a case of a patient with ALS in whom detection of advanced IAB during either on resting EKG or in a Holter registration was associated with intermittent paroxysmal atrial fibrillation episodes.
To our knowledge this is the first reported case with interatrial block and intermittent AF in a Holter registration in a patient with ALS.
A 70-year-old Caucasian men with a 5 year history of ALS was admitted to the hospital following worsening of an unsteady gait, vertigo, nausea, and vomiting. The patient was hypertensive under treatment. Moreover, no chest pain was reported during the last 3 months. He has experienced dyspneic episodes and intermittent use of a CPAP for breath amelioration. One year ago he underwent a cardiac ultrasound that demonstrated normal biventricular systolic function: He had degeneration of the mitral and aortic valves with no transvalvular gradients. There was left atrial dilatation and no pericardial effusion. In the last 2 months that patient was under treatment with ibersartan 150 mg daily, atorvastatin 20 mg one time daily and hydroclorothizide 25 mg once daily. Upon admission a chest X-ray demonstrated clear lung fields. His arterial blood gas revealed a PH 7.4 with PO2 77 mmHg and pCO2 38 mmHg. On neurologic examination, the patient had appropriate and fluent speech with no evidence of dysartria. Mental status examination was normal. There was normal upper and lower extremity strength. Deep tendon reflexes were normal and symmetric. His gait demonstrated severe ataxia. The patient's initial laboratory evaluation was unremarkable. Results of complete cell count, electrolytes and liver functions tests were within normal limits. Shortly after the initial evaluation the patient complained of a sudden onset of shortness of breath and palpitations. An initial EKG showed a sinus tachycardia 102 bpm. A pulmonary embolism was suspected. A pulmonary angio-TC was performed and was found to be negative. Additional laboratory analysis revealed normal thyroid function test results and C-reactive protein. Computed tomography of the head was normal. A bedside echocardiogram demonstrated normal cardiac chamber sizes with normal valvular function and contractility. Pericardial space was normal. In the department room a new resting EKG was obtained. A careful observation of the P wave morphology for duration and voltage in a resting EKG (Figure 1) which when magnified at 40 mm/mV and 25 mm/s paper velocity revealed an advanced interatrial block alteration (duration 124 ms, and the polarity was biphasic in leads II, III, aVF). Moreover, a holter registration (Figure 2 and Figure 3) was obtained revealing frequent intermittent episodes of paroxysmal atrial fibrillation. Moreover, a different duration of P wave was observed on the Holter measurement. Furthermore, during his hospitalization the patient received high dose methylprednisolone (1 gr/die) with gradual improvement in his neurologic abnormalities. Moreover, a prophylactic antiarrhythmic therapy with flecainide 100 mg 2 times daily for 5 days followed by 50 mg two times daily for the next period. Additionally, based on patient CHA2DS2VASc score equal to 4, apixaban 5 mg twice daily was also prescribed. Another holter EKG monitoring was performed 20 days later revealing no paroxysmal atrial fibrillation episodes.
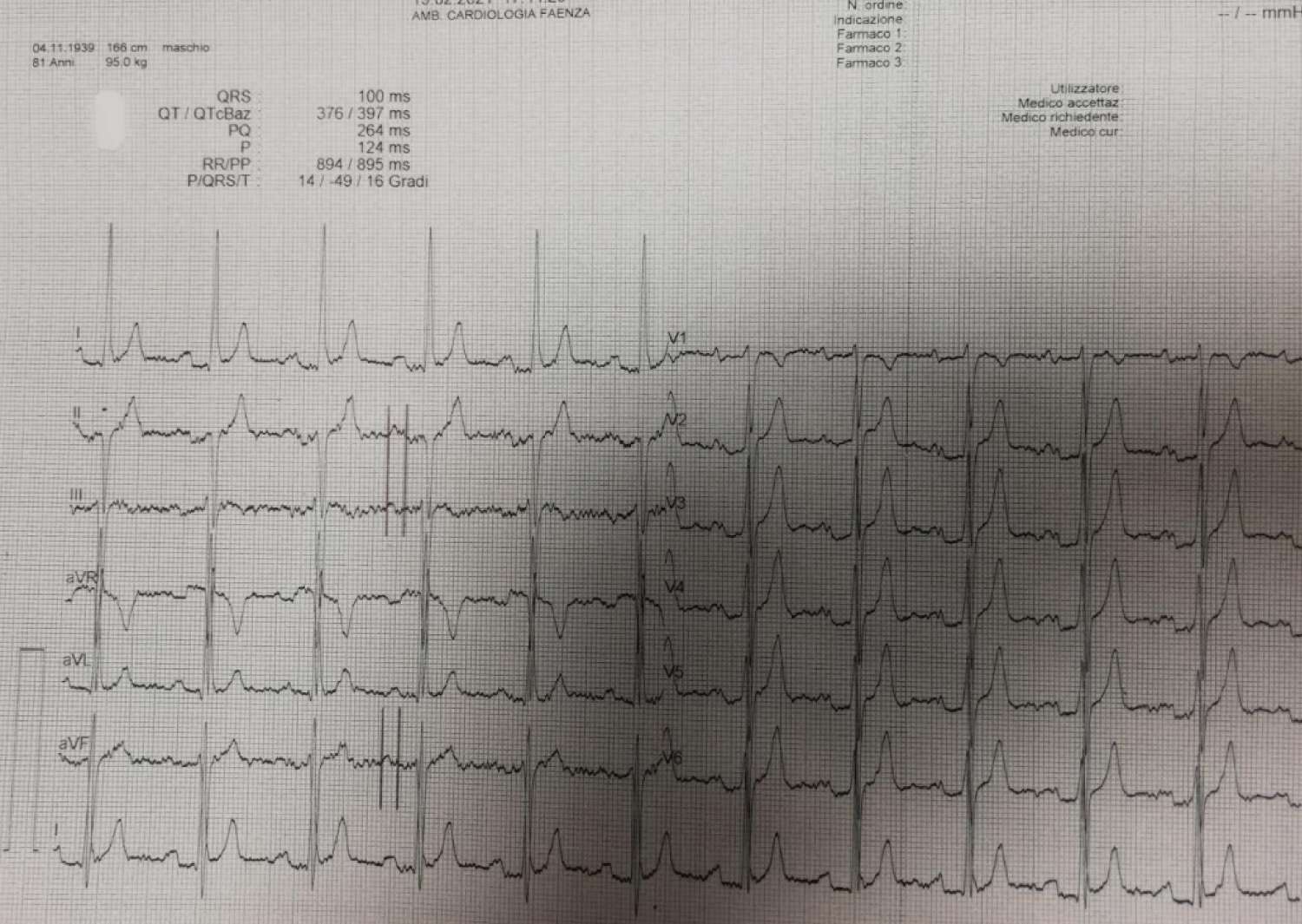 Figure 1: ECG: 12-lead ECG (with 40 mm/mV magnification and 25 mm/s paper velocity) at rest in the room. ECG shows sinus rhythm. P wave duration is increased (124 ms), P waves have a biphasic morphology in leads III and aVF, and a bimodal morphology in lead II, consistent with advanced IAB.
View Figure 1
Figure 1: ECG: 12-lead ECG (with 40 mm/mV magnification and 25 mm/s paper velocity) at rest in the room. ECG shows sinus rhythm. P wave duration is increased (124 ms), P waves have a biphasic morphology in leads III and aVF, and a bimodal morphology in lead II, consistent with advanced IAB.
View Figure 1
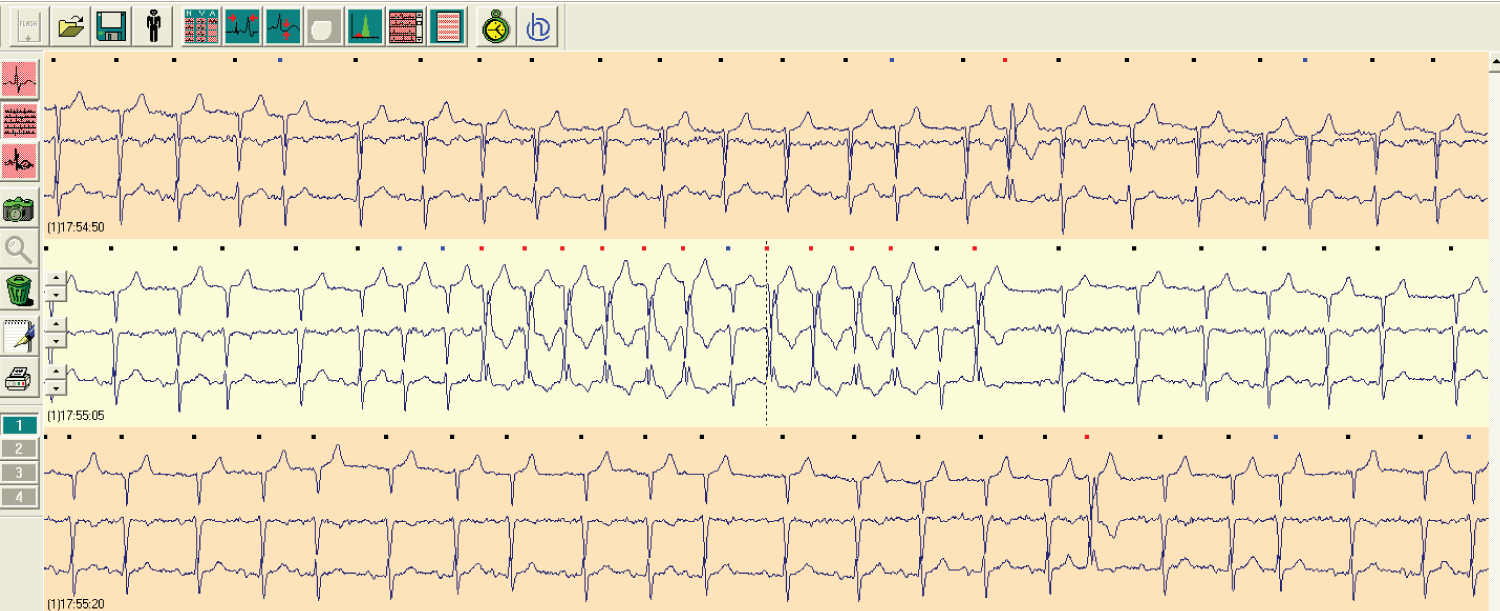 Figure 2: A short paroxysmal atrial fibrillation event on Holter EKG monitoring.
View Figure 2
Figure 2: A short paroxysmal atrial fibrillation event on Holter EKG monitoring.
View Figure 2
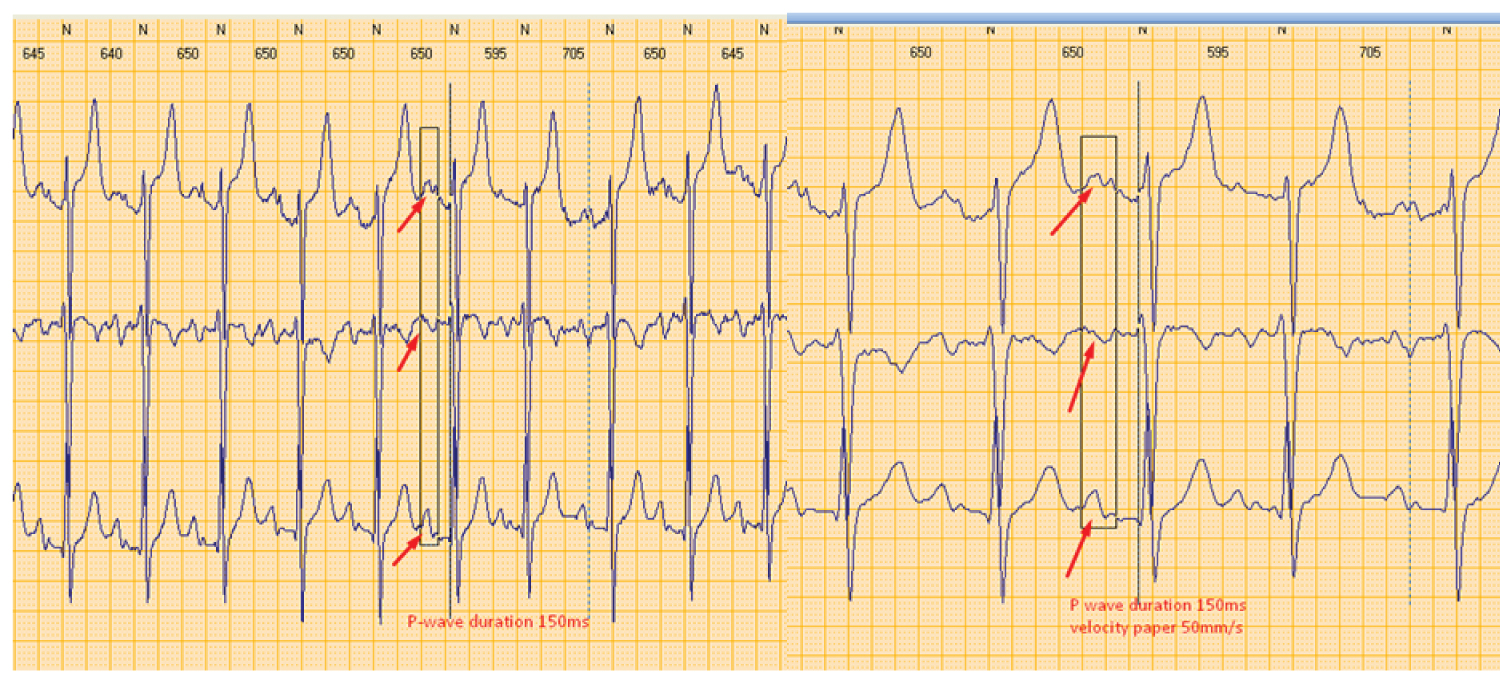 Figure 3: Interatrial block as it has been shown on Holter EKG monitoring. P wave measurements are obtained with semiautomatic application of Holter software at different velocity paper 25 mm/s (left part) and 50 mm/s (right part) in order to discriminate better P wave morphology.
View Figure 3
Figure 3: Interatrial block as it has been shown on Holter EKG monitoring. P wave measurements are obtained with semiautomatic application of Holter software at different velocity paper 25 mm/s (left part) and 50 mm/s (right part) in order to discriminate better P wave morphology.
View Figure 3
It is well accepted that IAB has been described to predict occurrence of newly onset and recurrent episodes of atrial fibrillation [6]. The prevalence of advanced and partial IAB is 1% and 9.7% respectively, in the general population and they are both associated with increased risk of AF [7]. Its prevalence increases with age, reaching a prevalence of 26% of advanced IAB and 20.1% of partial IAB in individuals > 100 years of age [11]. Aging is associated with a progressive increase in the degree of atrial fibrosis and modification in the cardiac conduction system, which can be associated with IAB. AF, atrial premature beats and runs of atrial arrhythmias are also associated with age and with IAB [12]. On the other hand, previous studies have reported that the association between ALS, a demyelinating disease of the brain stem, and abnormalities of cardiac conduction exists. Autonomic dysfunction begins with vagal release with enhanced sympathetic tone causing tachycardia. As patients later progress in ALS, sympathetic denervation occurs, followed by vagal predominance, leading to the development of bradycardia [13].
In a study examining the effects of vagal tone on the sinoatrial node and AV node, in patients with a history of syncope demonstrate sinus arrest or bradycardia. Increased vagal tone contributed to prolongation in the sinus cycle length [4]. The last finding may be caused by the presence of denervation edema. In our case an edema extension across Bachman branches may induce a conduction alteration process, resulting in IAB. In fact, a previous study involving CMR observed early gadolinium enhancement which in a disease with denervation, as the main muscular pathology, is likely to be associated with denervation edema [5]. Previous studies have shown that abnormalities in CMR were frequent in ALS, in particular the early gadolinium enhancement (77%) as a parameter of enhanced extracellular volume, that is either capillary leakage or fibrosis. The extension of that is difficult to define. Additionally, slowing of the sinoatrial node and inhomogeneous shortening of atrial refractory periods due to vagal stimulation in conjunction with fibrosis can facilitate IAB and develop AF. Another previous observation, using CMR and late gadolinium enhancement of the upper part of the septum, involving Bachmann's bundle which reported an association between IAB and atrial fibrosis [14].
As a result of the above condition an endothelium dysfunction may be present with a hypercoagulable state comparable to that present in AF [15,16] and a stagnant and sluggish left atrium favoring the appearance of stasis -induced thrombosis, especially in the left appendage, even in absence of supraventricular arrhythmias.
In the present case, holter EKG monitoring showed IAB and short intermittent episodes of atrial fibrillation. The causes of the different duration of the observed P-wave on Holter monitoring may be due to the dynamic registration of the Holter EKG, the semiautomatic measurement and the different day of registration. Moreover, a high CHA2DS2VASc-score was observed (equal to 4) and some studies suggest that patients with high CHA2DS2VASc-score and IAB have a high stroke risk, irrespective of the presence of AF [17,18]. These data support the notion that AF is a risk factor for ischemic stroke, but not necessarily the direct cause of it. On the other hand, in patients with ALS, cardiac arrhythmias are often untreated and symptoms are often attributed to pulmonary or psychiatric cause. But the degree of autonomic dysfunction and IAB may offer insight into the natural course of the disease. Covertible AF is relatively common in patients with ALS. To our knowledge, this is the first reported case of IAB occurring in ALS. Due to the high prevalence of AF in patients with IAB, the early recognition of advanced IAB by a high CHA2DS2VASc score in ALS patients may help to decide whether antiarrhythmic therapy or oral anticoagulation should be initiated by identifying the patients at a highest risk of AF. Although further randomized clinical trials are warranted in order to formalize such recommendation, IAB may occur in other neurodegenerative illnesses and may shed light into their disease progression as well as potential treatment. Flecainide, a class 1c antiarrhythmic agent is a strong blocker of persistent late Na+ channels in an open conformation. From previous studies using flecanide [19,20] have reported neuroprotective effects without promoting peripheral axonal degeneration but appear to modulate axonal function in ALS patients, stabilizing axonal excitability and reducing a decline in the neurophysiological index. Then, from a previous study, in flecainide treated patients maintained relatively stable peripheral membrane excitability over 32 weeks showing a significant biological activity at the peripheral nerve level [19]. Furthermore, flecainide appears to be effective in maintaining sinus rhythm after paroxysmal atrial fibrillation conversion [21].
In conclusion, ALS is a demyelinating disease of the brain stem, which sometimes is associated with cardiac conduction abnormalities and arrhythmias. IAB is an atrial conduction disturbance which may induce supraventricular arrhythmias and especially atrial fibrillation. SLA sometimes is associated with atrial arrhythmias especially due to denervation edema. The potential extension of this across Bachman branches may induce an interatrial conduction delay, presenting as IAB. EKG can help to discriminate patients at high risk needing early antiarrhythmic treatment and or potential anticoagulation therapy especially in those with a high CHA2DS2VASc score.
None.
Disclosure of funding received for this work from any of the following organizations: National Institutes of Health (NIH); Welcome Trust; and other(s) - None.
All authors made equal contribution.