Inflammation and platelet activation play a central role in the initiation and progression of the atherosclerosis process. The Platelet-to-lymphocyte ratio (PLR) is a new prognostic marker in coronary artery disease. The PLR is a significant independent predictor of long-term mortality after none-ST elevated myocardial infarction (NSTEMI). We aimed to evaluate the relationship between PLR and no-reflow (NR) in patients with NSTEMI.
The present study included 173 patients with NSTEMI. The patients were classified into two groups as follows: 115 patients in the NR group and 58 patients in the normal reflow group. NR was defined as coronary thrombolysis in myocardial infarction (TIMI) flow grade ≤ 2 after vessel recanalization with primary percutaneous coronary intervention. The PLR was calculated from the complete blood count.
The PLR values of the patients with NR were significantly higher than those of patients with normal reflow (108.6 (14.6-511.3) vs. 91.7 (17.2-225.3), p = 0.01). Also, neutrophil-to-lymphocyte ratio (NLR) was significantly higher in the NR group than the normal-reflow group (3.0 (0.3-16.5) vs. 2.3 (0.01-12.5), p = 0.02). The Correlation between the PLR and NLR was positive and significant (r = 0.68, p < 0.001).
This study showed that PLR is an independent predictor of NR in patients with NSTEMI.
Platelet-to-lymphocyte ratio, Angiographic no-reflow, Non-ST segment elevation myocardial infarction, Percutaneous coronary intervention
Acute coronary syndrome (ACS) is a leading cause of death worldwide, and patients with none-ST elevation myocardial infarction (NSTEMI) have a higher long-term mortality risk due to the prevalence of comorbidities and multi-vessel coronary artery disease (CAD) [1]. The diagnosis and prediction of significant CAD in NSTEMI can be challenging, and cardiac biomarkers, electrocardiography, symptoms, and cardiac risk factors are used to diagnose NSTEMI [2]. Inflammation and platelet activation plays a central role in the initiation and progression of the atherosclerosis process [3]. Also, increased platelet activation is associated with major adverse cardiovascular consequences [4]. On the other hand, lymphocyte count is inversely correlated with inflammation, and a low blood lymphocyte count is related to worse cardiovascular outcomes in patients with coronary artery disease [5]. The Platelet to lymphocyte ratio (PLR) was introduced as a potential marker for excess thrombotic activity [6] and inflammation in cardiac disorders [7]. The PLR has recently emerged as a significant predictor of major adverse consequences in cardiovascular disease [8]. Also, the PLR is a significant independent predictor of long-term mortality after NSTEMI [7]. However, there were no data regarding its predictive role for angiographic no-reflow (NR) in NSTEMI. Therefore, this study aimed to evaluate the relationship between PLR and angiographic NR in patients with NSTEMI.
From January 2017 to January 2018, a totally of 173 consecutive patients with NSTEMI, which underwent primary percutaneous coronary intervention (pPCI) within 24 hours of admission in Adıyaman University Training and Research Hospital, were enrolled into the study. CAD was defined as the presence of stenosis of at least 50% of the vessel diameter in any of the main coronary arteries, according to the American College of Cardiology/American Heart Association (ACC/AHA) lesion classification [9]. Significant CAD was defined as ≥ 70% stenosis in at least one major coronary artery or major side branch.
All of the patients were orally pretreated with 300 mg aspirin and 600 mg clopidogrel at the time of diagnosis before the intervention. Heparin (100 IU/kg) was administered before transportation to the catheterization laboratory or when the coronary anatomy was first defined. Baseline coronary angiography was performed using standard techniques (Siemens Axiom Artis zee 2011; Siemens Healthcare, Erlangen, Germany). Each coronary artery was displayed in at least two different plane images. Iopromide, as a contrast agent (Ultravist-370, Bayer Schering Pharma, Germany), was used in all subjects. All of the pPCI procedures were performed with a standard femoral approach with a 6-French guiding catheter (Launcher; Medtronic, Minneapolis, Minnesota, USA). An angiographic evaluation was made by visual assessment. After administration of unfractionated heparin conventional wire crossing, direct stenting was implanted whenever possible, and if necessary, balloon predilatation was carried out. For each procedure, interventional success was defined as reducing obstruction and stenosis to 30% of the infarct-related artery just after primary angioplasty.
The Thrombolysis in Myocardial Infarction (TIMI) flow grade was evaluated by an experienced interventional cardiologist, blinded to the patients clinical data, using quantitative cardiovascular angiographic software (Axiom Sensis XP; Siemens, Munich, Germany). The patients were divided into two groups based on the post-intervention TIMI flow grade: normal-reflow group and NR group. Normal-reflow was defined as post-intervention TIMI grade 3 flow. NR is defined as an inability to restore blood flow to previously ischemic myocardium even after the return of infarct-related epicardial coronary artery patency due to microvascular obstruction. NR group consisted of both patients with angiographic no-reflow [10] (defined as post-intervention TIMI grade 0-1-2 flow). The syntax score (SX score) for each patient was calculated by scoring all coronary lesions with diameter stenosis of at least 50%, in vessels at least 1.5 mm, using the SX score algorithm, and is available on the SX score website (Available at http://www.syntaxscore.com).
Blood samples are collected from the antecubital vein by an atraumatic puncture and are sent to the laboratory for analysis within 1 hour after collection. In all patients, blood samples were drawn on admission. Common blood counting parameters stored in citrate-based anti-coagulated tubes were measured by Coulter LH 780 Hematology Analyzer (Beckman Coulter Ireland Inc, Mervue, Galway, Ireland) within 5 minutes of sampling. Glucose, creatinine, lipid profile, and cardiac enzymes were determined by standard methods. PLR was calculated as the ratio of platelet count to lymphocyte count. The neutrophil to lymphocyte ratio (NLR) was calculated as the ratio of neutrophil count to lymphocyte count. The inclusion criteria of the study were as follows: Documented gradual rise and fall of serum troponin levels with a peak value of > 0.5 ng/ml. An NSTEMI diagnosis was based on elevated cardiac enzymes with typical chest pain and electrocardiographic changes, the notable absence of ST-segment elevation, suggestive of myocardial ischemia [11]. Typical chest pain was evaluated as follows: More than 20 minutes (min) in duration, new-onset angina, and an increase in its frequency and duration or severity. A cardiologist confirmed the NSTEMI diagnosis. Exclusion criteria included cardiogenic shock on admission, serious arrhythmias with hemodynamic instability, history of coronary artery bypass greeting, active infections, systemic inflammatory disease history, known malignancy, hematologic disorders, liver disease, and renal failure.
From medical records, we obtained demographic information, cardiovascular history, and risk factors for CAD, and treatment received during the in-hospital period. Laboratory studies, including Platelet and lymphocyte counts, were analyzed. Clinical information included data on systemic hypertension (HTN), diabetes mellitus, dyslipidemia, smoking, and previous history of CAD. Diabetes was based on a fasting blood sugar level ≥ 126 mg/dL or use of an anti-diabetes medication. Hypertension was reported for systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg or use of antihypertensive agents. Hyperlipidemia was reported for total cholesterol ≥ 200 mg/dL, low-density lipoprotein (LDL) level ≥ 130 mg/dL or use of cholesterol-lowering medication. Smoking included active or previous (> 10 pack-years) tobacco use. 12-lead electrocardiography (ECG) was recorded in each patient just after hospital admission. At 24 h to 72 h after revascularization, transthoracic echocardiography study was performed with a 3.5-MHz transducer to all patients (Vivid 3; GE Medical System, Horten, Norway). Simpson's method was used to assess left ventricular ejection fraction (LVEF), as recommended by the American Society of Echocardiography [12].
The study protocol was approved by the local ethics committee of the Adıyaman University Training and Research Hospital, and each patient provided written, informed consent.
Data were analyzed with SPSS software version 20.0 for Windows (SPSS Inc, Chicago, Illinois). The Kolmogorov-Smirnov test was used to verify that continuous variables were normally distributed. Normally distributed variables were expressed as mean ± standard deviation (SD), while non-normally distributed variables were expressed as median with interquartile range (IQR). The categorical variables are presented as percentages. Differences between two groups were evaluated with Student's unpaired t-test or the Mann-Whitney U test for parameters with a normal or non-normal distribution. The frequencies of nominal variables were compared using Fisher's exact test and chi-square test. The Pearson test was used for correlation analysis after testing variables for normality. Statistical significance was defined as P < 0.05.
The demographic and clinical data of the study population are presented in Table 1. Laboratory findings and angiographic data are in Table 2. There were 115 patients in the NR group (63.13 ± 10.77 and 57% male) and 58 patients in the normal-reflow group (mean age 63.51 ± 10.43 and 65% male). No difference was found between the groups regarding HT, DM, Smoking, hyperlipidemia, history of PCI, and history of MI, whereas the Syntax Score was significantly higher in the NR group. The PLR and NLR was significantly higher in the NR group than the normal-reflow group (108.6 (14.6-511.3) vs. 91.7 (17.2-225.3), p = 0.01; 3 (0.3-16.5) vs. 2.30 (0.01-12.5), p = 0.02; respectively) (Table 2). Box plot graph was used to show PLR values in NR and normal-reflow groups (Figure 1). The hemoglobin was significantly higher in the NR group than the normal-reflow (14.27 ± 1.86) vs. (13.44 ± 1.99), p = 0.008. The Correlation between the PLR and NLR was positive and significant (r = 0.68, p < 0.001) (Figure 2).
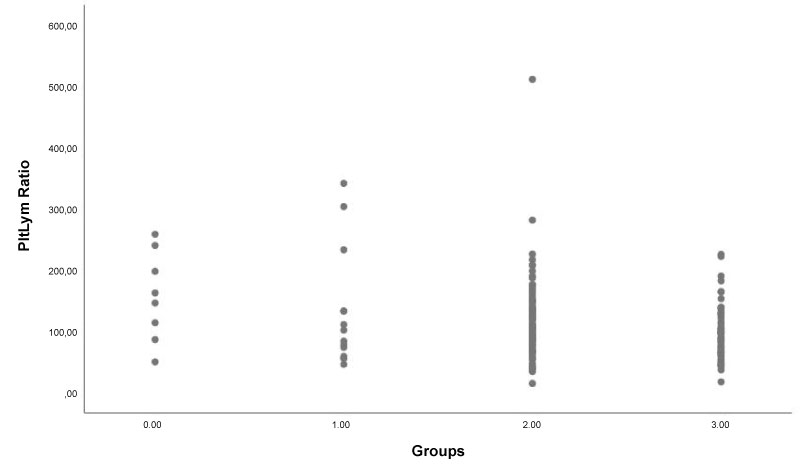 Figure 1: Box plot graph shows platelet-lymphocyte ratio (PLR) values in angiographic no-reflow (TIMI-0,1,2) and normal-reflow (TIMI-3) groups.
View Figure 1
Figure 1: Box plot graph shows platelet-lymphocyte ratio (PLR) values in angiographic no-reflow (TIMI-0,1,2) and normal-reflow (TIMI-3) groups.
View Figure 1
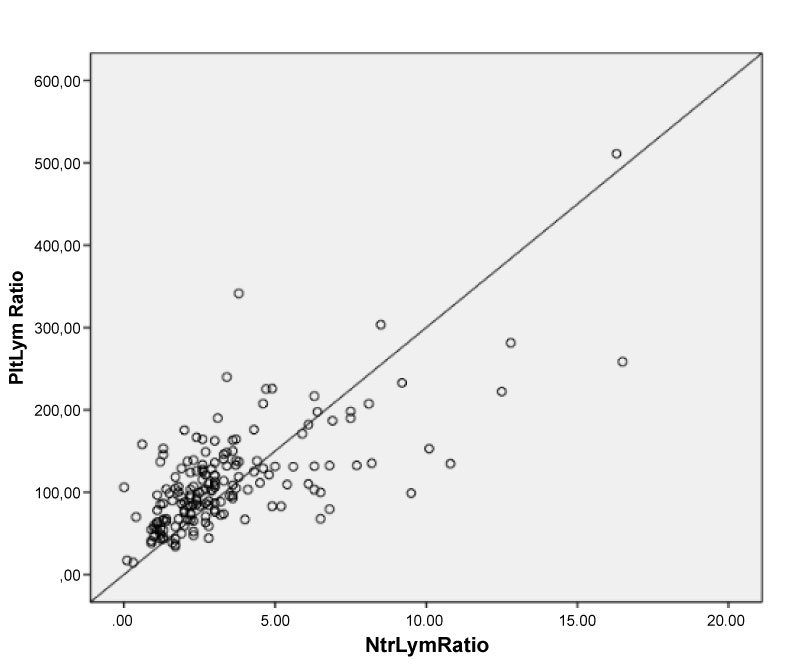 Figure 2: The Correlation between the PLR and NLR was positive and significant (r = 0.68, p < 0.001).
View Figure 2
Figure 2: The Correlation between the PLR and NLR was positive and significant (r = 0.68, p < 0.001).
View Figure 2
Table 1: Baseline clinical characteristics of the study population. View Table 1
Table 2: Angiographic and laboratory measurements of study groups. View Table 2
This study demonstrated two significant findings in patients with NSTEMI undergoing pPCI. First, the PLR and NLR on admission were significantly higher in the post-intervention NR group. Second, there was a positive and significant correlation between the PLR and NLR in patients with NSTEMI.
NSTEMI arises from the interaction of both inflammatory and thrombotic pathways; platelets and lymphocytes play an essential role during this process. The impact of inflammation has been shown both in the initiation and in the progression of atherosclerosis, and inflammation also plays an active role during the atherosclerotic process [13]. Platelet-induced inflammatory processes might play a pivotal role in atherothrombosis [14]. The release of various mediators such as IL-1 and IL-3 during the pro-inflammatory state causes megakaryocyte proliferation and an increase in circulating platelet count [15]. Thus, increased platelet counts may indicate enhanced thrombocyte activation and a prothrombotic state [16]. Another study reported that lymphocytopenia was independently related to mechanical complications and mortality in patients with acute myocardial infarction (AMI) [17]. In response to physiologic stress during myocardial ischemia/infarction, there is a release of cortisol, and high cortisol leads to lymphopenia [18]. Lymphopenia shows a high inflammatory process [19]. Ommen, et al. have documented a decrease in the total and relative numbers of circulating lymphocytes during AMI and advanced congestive heart failure (HF) [20].
Moreover, a low lymphocyte count is associated with worse outcomes in NSTEMI patients [18]. Dogan, et al. showed that low lymphocyte counts are independent predictors of the NR phenomenon [21]. On the contrary, Yuksel, et al. did not find a correlation between lymphocyte and severity CAD [22]. Eventually, both thrombocytosis and lymphopenia correlate with the degree of systemic inflammation.
The increased amount of platelets can show elevated thrombocyte activation and plays an active role in the platelet-fibrin formation, and pathogenesis of ACS [23]. Stissing, et al. reported that patients with pathologically increased platelet counts have an elevated risk of thrombotic complications [24]. Recent studies have demonstrated that increased platelet count and decreased lymphocyte count are associated with bad prognosis in patients with CADs [4,25]. Also, platelets contribute to the process of restenosis and, potentially, cardiac mortality after unstable angina/NSTEMI [26]. Muller, et al. showed that platelet counts predict the long term mortality in NSTEMI [27]. On the other hand, high PLR is a significant predictor of poor coronary collateral circulation development in patients with NSTEMI [28]. Akdag, et al. found that there was a positive correlation between PLR and the severity of CAD in patients with NSTEMI [28]. Also, PLR predicts the left ventricular systolic dysfunction in NSTEMI [29]. Moreover, PLR is a new predictor of major adverse cardiovascular outcomes [30]. The advantage of the PLR that reflects the activity of both the hemostatic and the inflammation pathways. Increased PLR is an independent predictor of long-term mortality in patients with NSTEMI [7]. Also, PLR inversely related to infarct-related artery patency [31] and associated with increased risk of in-hospital worse outcomes and six months of all-cause mortality in patients with STEMI after PCI [32]. Kurtul, et al. found that PLR was an independent predictor of the prevalence of severe coronary artery lesions, and an in-hospital predictor mortality in patients with ACS [33].
NR remains a severe complication of PCI of patients with AMI after revascularization [34]. NR has a strong negative impact on clinical outcome and independently associated with early post-infarct complications, late repeat hospital stays for heart failure, and mortality [35]. Also, angiographic NR after PCI is associated with reduced myocardial salvage, and larger infarct size [36]. NR is a multifactorial phenomenon, and five mechanisms have been recognized by Niccoli [37]: (I) pre-existing microvascular dysfunction; II) distal micro-thrombo-embolization; III) ischemic injury; IV) reperfusion injury and V) individual susceptibility. Thus, Intracoronary thrombus and high thrombus burden have an essential role in the occurrence of NR [38]. The rush of platelets and neutrophils that follows reperfusion may lead to the formation of neutrophil-platelet aggregates that plug the microcirculation and reperfusion-related injury [39]. When embolic microspheres block > 50% of coronary capillaries, Myocardial perfusion starts falling, and distal micro-thrombo-embolization during PCI is a predominant cause of NR [37]. PLR is not only a biomarker which reflects acute inflammatory activity but also an indicator of the thrombus burden causing embolization. In the present study, we showed that NR is related to systemic inflammation, and early detection, preventive measures, and treatment of NR may alter the outcome of PCI in patients with NSTEMI. Also, PLR and NLR were higher in the NR group with NSTEMI in the present study. The NLR represents the balance between neutrophil and lymphocyte levels in the body and can be an indicator of systemic inflammation [3].
We demonstrated that PLR was not only higher in the NR group than the normal reflow group; it also emerged as an independent predictor of NR after pPCI in NSTEMI patients. PLR is a simple and readily available biomarker in clinical settings. Thus, PLR can be used as a practical, inexpensive, and important tool for predicting the NR in patients with NSTEMI. PLR may also enable risk stratification and selection of a treatment strategy in patients with NSTEMI before or during coronary interventional procedures. We suggest the need for further evaluation of PLR as a predictor of NR phenomena in prospective studies.
This present study has some limitations. This study based on a relatively small group of patients and was a single-center study. Coronary blush grade and extent of coronary thrombus parameters were not analyzed in the coronary angiography. Another study limitation was infarct size and/or ischemic damage biomarkers (myoglobin, ischemia modified albumin and cardiac fatty acid-binding protein) that we could not measure in our institution. Lack of other established inflammatory markers, such as C-reactive protein, interleukin-6, and tumor necrosis factor-α, is another limitation of the study. The multi-center prospective and randomized studies are needed to confirm our findings.
All authors have no declarations of interest to report.
RA, AS performed the research and analyzed data. AS, EA, AA provided clinical data. RA, AS, EA wrote the paper, and all authors critically reviewed and edited the paper.
None.
None.