Prosthetic thrombosis, Biological valve prosthesis, Transoesophageal echocardiogram, Cardiac surgery
Bioprosthetic valve thrombosis (BPVT) is considered to be a rather unusual complication among this type of prosthesis. However, several recent data show the importance of a careful diagnosis and the adequate discrimination with respect to other possible complications, which may be the cause of an underestimation of its incidence. We present the case of a woman with biological prosthesis thrombosis after a mechanical prosthesis thrombosis. It is remarkable the transoesophageal echocardiography's contribution to the diagnosis of this entity. As well as the need for more evidence and recommendations in relation to the optimum treatment and its prevention.
We present the case of a 72-year-old woman with history of dyslipidemia, mellitus diabetes, hypothyroidism and a permanent pacemaker. She had a mechanical mitral prosthesis implanted because of rheumatic valve disease. A few years later, however, she had to undergo a second surgery as the prosthesis was thrombosed (Figure 1 and Figure 2). The mechanical prosthesis was replaced and a biological one implanted. Treatment with acenocumarol was maintained due to atrial fibrillation. A month after the second surgery she was hospitalized for decompensated heart failure and thrombosis of the biological mitral prosthesis was diagnosed.
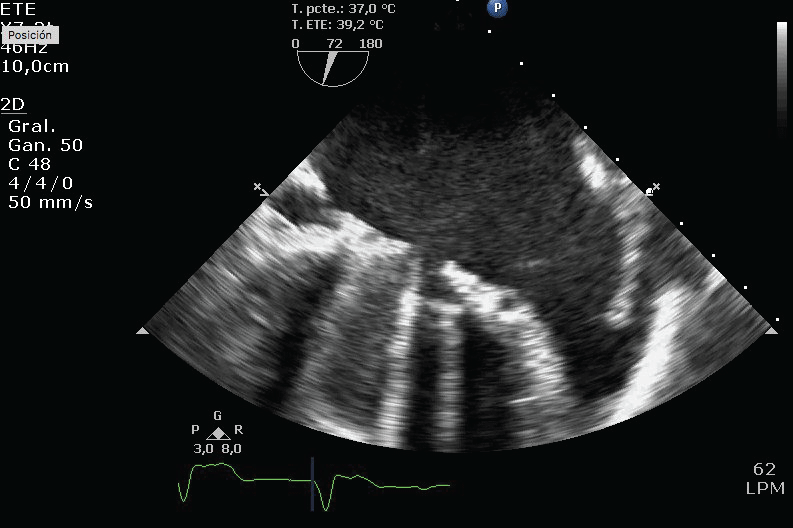 Figure 1: Mechanical prosthesis thrombosis. Lack of opening of one disk because of the thrombus. View Figure 1
Figure 1: Mechanical prosthesis thrombosis. Lack of opening of one disk because of the thrombus. View Figure 1
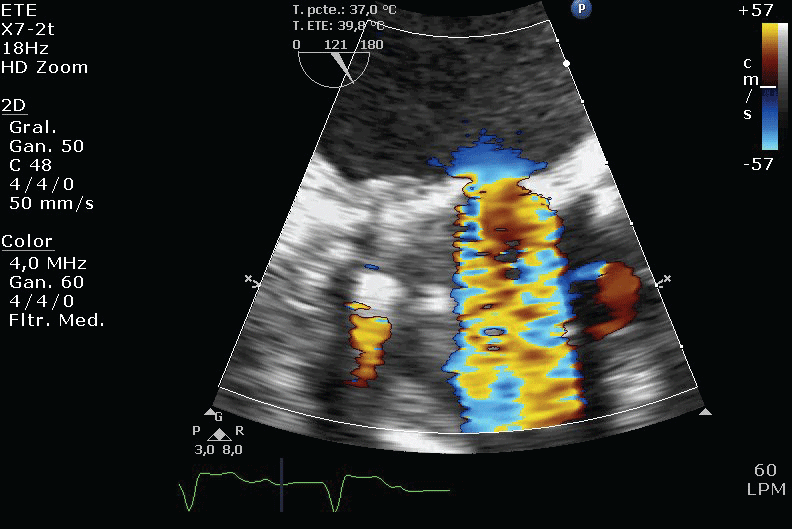 Figure 2: Turbulent flow trough the thrombosed mechanical prosthesis. View Figure 2
Figure 2: Turbulent flow trough the thrombosed mechanical prosthesis. View Figure 2
Transthoracic echocardiogram showed that the gradients of the prosthesis were higher than in previous exam and there was no fever, anemia nor hyperdynamic state that could justify that fact. It also showed moderate pulmonary hypertension. A transoesophageal echocardiogram was then conducted, and we confirmed the lack of movement of one of the veils (Figure 3 and Figure 4) with a thickening on its ventricular face, suggesting a thrombus. It also showed a diminished effective area of 1 cm2 and turbulent flow through the valve (Figure 5).
 Figure 3: Thrombus on the ventricular face of biological prosthesis veil. View Figure 3
Figure 3: Thrombus on the ventricular face of biological prosthesis veil. View Figure 3
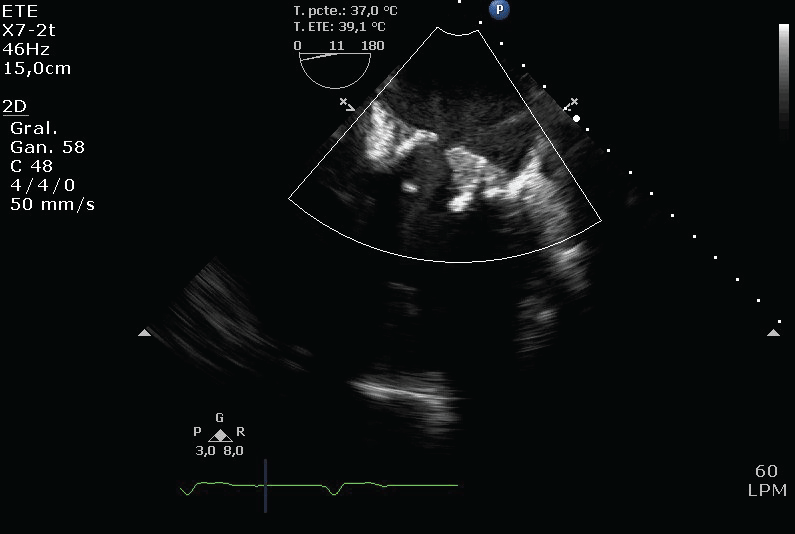 Figure 4: One of the veils of the biological prosthesis stuck in the close position. View Figure 4
Figure 4: One of the veils of the biological prosthesis stuck in the close position. View Figure 4
 Figure 5: Turbulent flow through the bioprosthesis valve. View Figure 5
Figure 5: Turbulent flow through the bioprosthesis valve. View Figure 5
Thrombi have to be differentiated from fibrous pannus, but this sort of complication appears longer after surgery. Strands or sutures are also difficult to differentiate from a thrombus, but they are finer filamentous mobile echoes and they do not alter the gradients. Furthermore, in this case the thickening was not seen in previous echocardiograms after surgery. On the other hand, there were no signs of infection and normally infective vegetations have an independent mobility from the involved veil. Taking all of this into account we thought the most probable diagnosis was a new episode of prosthesis thrombosis, on a biological one this time.
BPVT is rather uncommon, being more frequent during the first three months after surgery. In the case that concerns us, it was the second episode of thrombosis under anticoagulant therapy. We investigated the possibility of a hypercoagulability disorder, and the patient was found to be heterozygous for factor V Leiden.
Though treatment options for mechanical prosthetic valve thrombosis are surgery, thrombolytic therapy and anticoagulation. When we talk about BPVT, anticoagulation therapy usually has satisfactory results. As any surgery would have been a third intervention, we opted for medical management. Given the patient's hemodynamic stability we started a strict anticoagulation treatment with intravenous heparin as well as antiplatelet therapy. A few days later, the thrombus had disappeared, the valve mobility was normal, and the gradients had diminished to standard ranges. She was discharged on treatment with aspirin and acenocumarol, with target INR levels between 2.5 and 3.5, and strict echocardiographic control.
BPVT has always been considered a rather unusual complication among this type of prosthesis. Nevertheless, recent studies suggest that it may be underestimated and that it could be more prevalent than previously reported.
Unlike mechanical prostheses, the incidence of thrombosis in biological prostheses is estimated to be less than 1% in symptomatic cases, reaching up to 14% in subclinical ones, with a peak around 1 or 2 years after implantation [1].
Possible factors that may be associated with its development are: The mitral position, a history of previous thrombotic episodes and the cessation of anticoagulant treatment [2]. Another data that has been newly associated with this complication is systolic dysfunction of the left ventricle, and even the type of tissue used in the manufacture of the prosthesis [3]. The patient in our case presented atrial fibrillation and the previous thrombotic event as risk factors. The result of heterozygosis for factor V Leiden without any other associated abnormality carries a very low risk of thrombosis, and it affects specially to smoking patients [4]. Other coagulation disorders that must be rule out are antithrombin deficiency, protein S and protein C deficiency and the prothrombin gene mutation.
Clinically, BPVT can show up as an accidental finding in asymptomatic patients on their routine echocardiographic control. But it can also trigger a valve obstruction driving to congestive heart failure or even cardiogenic shock or be the origin of a systemic or pulmonary thromboembolic event.
Transthoracic echocardiography can help us with this entity diagnosis, but biological valves can demonstrate non-uniform opening of the mitral valve leaflets, which is normal, so that we usually need further research. Transesophageal echocardiography remains the gold standard. A raise in transvalvular gradient higher than 50% of previous studies, an increase in veil thickness or an abnormal mobility of it [5] are echocardiographic findings suggestive of thrombosis. Also, the emergent 4D computerized tomography seems like a promising technique regarding the diagnosis of subclinical prosthesis thrombosis, but there is still a need for more experience on this ground [1]. Other clinical factors that support this diagnosis are atrial fibrillation and low levels of INR. Nevertheless, there are many times when BPVT is difficult to differentiate from other possible complications which require a different management, such as fibrous pannus or infective vegetations, or even from strands and sutures. That is why it is important to investigate the medical record, search for previous exams and carry out supplementary tests such as laboratory tests, or as an example, cultures and PET CT scans to rule out endocarditis.
With respect to the treatment strategy of BPVT, it depends on clinical presentation, the patient's hemodynamic status, the presence of valvular obstruction and the prosthesis location [6]. In hemodynamically unstable patients, obstructive thrombosis or congestive heart failure, surgery is usually preferred as the first choice. However, if there is surgical contraindication, high surgical risk or in right-sided prosthesis fibrinolysis could be considered. The newly "valve in valve" technique can also be taken into account in high surgical risk patients. On the other hand, in minimally obstructive valves with small thrombi (< 0.8 cm2) medical approach with anticoagulation treatment can be conducted [7]. Thrombus formation on biological valves has been repeatedly demonstrated in TAVR patients, where treatment consisted of augmented anticoagulation. In most cases, there is a clinical and echocardiography Cal improvement with normalization of prosthetic gradients after 2 months of anticoagulant treatment [1]. It is unclear thought how long the duration of this treatment should be after a BPVT, being important to bear in mind the risk of bleeding of the patient as well as the risk of recurrence of the event. There is also another lack of information regarding the novel oral anticoagulant agents and their place in treatment and prevention of BPVT.
Because of the arrival of thranscatheter valve replacement as well as a growing elderly population that undergoes surgical interventions, the number of biological prosthesis implanted is on the rise. That is why knowing the possible complications that may appear is nowadays a priority.
In the exposed case implanting a biological prosthesis was thought to diminish the risk of thrombosis, but thrombosis developed. That must lead us to think of BPVT as not such an uncommon complication. It is important to have this possible diagnosis in mind, given the fact that a proper and early treatment may avoid a dreadful prognosis. We must also highlight the essential role of echocardiography's contribution to making this diagnosis and the still existing gaps in evidence about the appropriate management of this entity, such as the duration of anticoagulation therapy and the position of novel oral anticoagulants agents in BPVT.