Thrombotic thrombocytopenic purpura (TTP) is a rare and life-threatening thrombotic microangiopathy (TMA) characterized by microangiopathic hemolytic anemia, severe thrombocytopenia, and organ ischemia linked to disseminated microvascular platelet rich-thrombi. TTP is specifically related to a severe deficiency of ADAMTS13, a cleaving protease for von Willebrand (vWF). TTP is occurring in between 1 and 13 cases per million people depending on geographic location, and 1 in 25,000 pregnancies. We present 40-yr-old parturient with TTP who underwent spinal anesthesia for cesarean delivery at 37 weeks of gestation. Treatment for our case included acetylsalicylic acid, methylprednisolone and administration of fresh frozen plasma (FFP).
We emphasize the importance of a multidisciplinary team approach to succeed the best outcome for this patient. Literature is discussed.
Thombotic thrombocytopenic purpura, ADAMTS13 deficiency, Anesthetic management, Spinal anesthesia, Cesarean delivery
ADAMTS-13: A Disintegrin and Metallo Proteinase with a Thrombospondin Type 1 Mot If, Memder 13; AFLP: Acute Fatty Liver of Pregnancy; ASRA: American Society of Regional Anesthesia and Pain Medicine; ECG: Electrocardiogram; ESA: European Society of Anesthesiology; FFP: Fresh Frozen Plasma; HBP: High Blood Pressure/Hypertension; HELLP: Haemolysis, Elevated Liver Enzymes, Low Platelets; HUS: Hemolytic Uremic Syndrome; LDH: Lactate Dehydrogenase; MAHA: Microangiopathic Hemolytic Anemia; NIBP: Non-Invasive Blood Pressure; PLT: Platelet; SpO2: Oxygen Saturation; TMA: Thrombotic Microangiopathy; Attp: Acquired Type of TTP; Cttp: Congenital Type of TTP; TTP: Thrombotic Thrombocytopenic Purpura; Vwf: Von Willebrand Factor
TTP is characterized by formation of platelet-rich thrombi in both the arterial and capillary microvasculature, resulting in thrombocytopenia and micro angiopathic hemolytic anemia (MAHA). The cause of TTP is a deficiency of ADAMTS13, a protease that disrupts Vwf multimers into smaller pieces. The deficiency leads to accumulation of ultra large vWF multimers in plasma resulting in the formation of blood clots in the microcirculation.
TTP is classified as congenital (cTTP), where biallelic mutations of ADAMTS13 occur, and acquired or idiopathic (aTTP), the most common, where auto antibodies anti-ADAMTS13 exist. Pregnancy can trigger TTP, although diagnosis can be difficult since clinical and laboratory findings can be very similar to those of preeclampsia and HELLP syndrome. TTP can also be related to drugs, chemotherapy, and hematopoietic stem cell transplant. Clopidogrel, ticlopidine, cyclosporinA, mitomycin C, have been associated with triggering TTP [1].
We present the anesthetic management of a parturient with TTP presented for cesarean section. Written consent was taken from our patient for publication of this case report.
A 40-year-old pregnant patient at 37 weeks of gestation, BMI 27.88 (67 kg weight, 155 cm height) diagnosed with TTP, submitted to our hospital for a scheduled cesarean section. She was first diagnosed with TTP, at the age of 37, after a miscarriage due to hemorrhage at the 22th week of gestation.
Patient was first treated with plasmapheresis, followed by rituximab for one year. Her medical history also included hypothyroidism, treated with levothyroxine sodium 125 μg once per day.
During pregnancy the patient was treated with prophylactic regimen of 100 mg of acetylsalicylic acid once per day and methyl prednisolone tab 16 mg at the 21st week of gestation, as well as with three units of FFP every two days. After the 30th week of gestation, two units of FFP were administered. At the 36 + 3th week of gestation both acetylsalicylic acid and FFP administration have been stopped. Platelet (PLT) counts were closely monitored during pregnancy, as shown in Figure 1. She remained hemodynamically stable throughout the pregnancy.
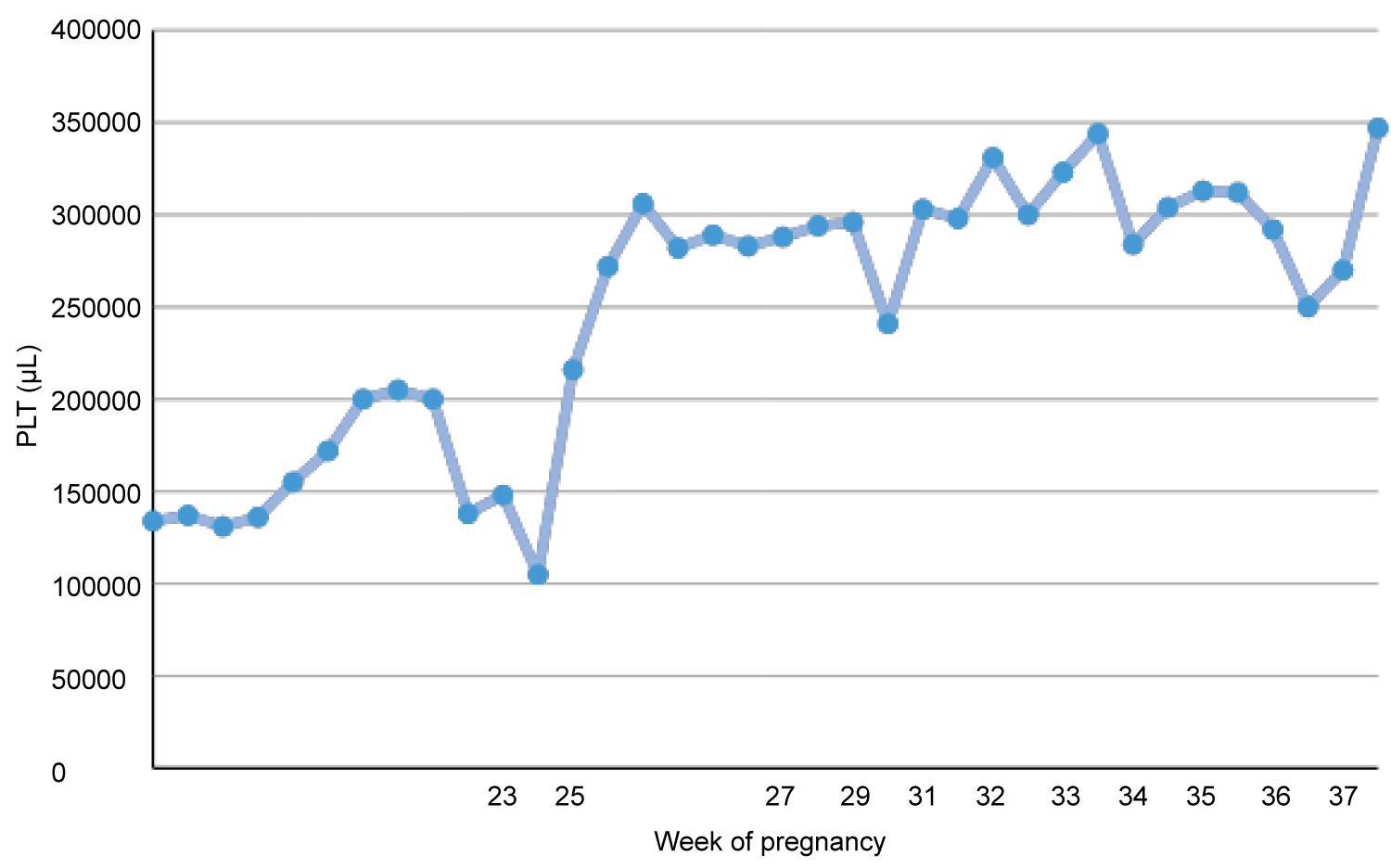 Figure 1: Variation of PLT counts throughout pregnancy.
View Figure 1
Figure 1: Variation of PLT counts throughout pregnancy.
View Figure 1
On the day of the surgery vital signs were: noninvasive blood pressure (NIBP) of 123/60 mmHg, heart rate (HR) of 84 bpm with sinus heart rhythm, and SpO2 of 98% at room air. Laboratory tests the day before were within normal limits; hemoglobin 11.8 g/dL, hematocrit 33.5%, PLT 347.000 μL, International Normalized Ratio (INR) 0.84, prothrombin Time (PT) 9.8 sec, activated partial thromboplastin Time (aPTT) = 17.6 sec. Fasting status was 6 hours.
On the arrival in the operating theater, a 17G and a 18G venous catheter were installed on the left forearm and basic monitoring was attached (ECG, NIBP, SpO2). Throughout the operation urine output was also monitored. Before any anesthetic intervention 500 ml of Ringer's Lactate solution and 100 mg hydrocortisone were administered, according to endocrinologic recommendations. Spinal anesthesia was performed with the patient in the left lateral position, under sterile conditions. After Infiltration of local anesthetic (4 ml Lidocaine 1%) in the L3-L4 inter space, spinal anesthesia was performed with first attempt, uncomplicated (25G Whitacre Pencil Point). With a dose of 10.5 mg of hyperbaric bupi vacaine 5% and 15 mcg fentanyl the level of the sympathetic blockade was tested at T4 dermatome.
Simultaneously with the performance of spinal anesthesia, an infusion of a dilute norepinephrine solution (8 mcg/ml) in a standard rate of 15 ml/h and a rapid 1,000 mL crystalloid (Lactated Ringer's solution) cohydration were intravenously administered. The cesarean section was performed without the occurrence of hypotension (blood pressure 103/59 mmHg, HR 80 bpm, SpO2 98%).
A female neonate was delivered (2450 gr) with Apgar score 8. After birth, the norepinephrine infusion was stopped; an oxytocin infusion (20 UI/1000 ml Ringer's Lactate) was administered as an uterotonic. Adjuvants been used included: cimetidine 200 mg, metoclopramide 10 mg, cefoxitin 2 gr, ondansetron 4 mg, paracetamol 1 gr, dexketoprofen 50 mg.
The surgery was completed in 55 minutes, without any complications. The parturient was transferred to the post anesthesia care unit (PACU) for post-operative monitoring, where she remained for 60 minutes. She was transferred back to the ward after full mobilization of legs and stable vital signs.
TTP is characterized by formation of thrombi in the microvasculature of affected organ. These thrombi consist of PLT and ultra large vWF multimers. The net cause is a deficiency of ADAMTS13, leading to inability of disruption of vWF multimers [1]. In adults it is most often associated with acquired deficiency of ADAMTS13 due to auto-antibodies, whereas TTP in children is most often associated with a hereditary autosomal recessive severe deficiency of ADAMTS13 [2].
During pregnancy, thrombocytopenia is the second most common abnormality of the complete blood count. Thrombocytopenia is defined as a platelet count below 150,000 μL, and the causes can be pregnancy-related or non-pregnancy-related, as shown in Table 1 [3].
Table 1: Causes of thrombocytopenia during pregnancy and relative frequencies. View Table 1
The incidence of thrombocytopenia during pregnancy or in the immediate postpartum period is approximately 5% to 10%. There are a variety of causes being related to a low platelet count and it may be underlying a coexisting systemic or gestational disorder [4,5].
There is also a classification of thrombocytopenia's etiology according to the trimester and platelet count, as shown in Table 1. Most common causes of thrombocytopenia during the third trimester are gestational thrombocytopenia and pre-eclampsia/HELLP syndrome [6] (Table 2).
Table 2: Classification of thrombocytopenia's etiology according to the trimester PLT count. Others include: Bone marrow failure, antiphospholipid antibody syndrome, autoimmune conditions. View Table 2
Gestational thrombocytopenia at a rate of 75% is the most common cause of thrombocytopenia in pregnancy. The most common form of thrombocytopenia during pregnancy is gestational thrombocytopenia, due to dilutional changes, at a rate of 75% of cases. Gestational thrombocytopenia arises in 5-8% of all pregnancies. PLT count is usually above 130,000-150,000 μL. There is no increase of the bleeding risk, needs no treatment and it does not affect the delivery method [3,7]. A 15-20% encounters hypertensive disorders; 3-4% is due to immune disorders while the 1-2% is due to other rare causes [7].
The incidence of TTP in the general population is 3-11 cases per million people. It is more frequent in women, with its peak in the fifth decade in the United States, third decade in Europe [8,9]. TTP occurs in 1:25,000 pregnancies, while pregnancy can trigger a 5-25% of TTP cases [7,10]. During pregnancy plasma vWF increases, and can trigger TTP in otherwise asymptomatic patients with specific ADAMTS13 mutations [11]. There is also a correlation between the type of TTP and the period of manifestation. ATTP presented more frequently in the postpartum period compared with the cTTP that presents more frequently in the third trimester [2,12].
TTP can be described as a complex of five signs including thrombocytopenia, microangiopathic hemolytic anemia, fever, renal failure and neurologic manifestations. Not all patients with TTP have all five signs. Patients usually report an acute onset of symptoms related to central nervous system (seizures, paresthesias, visual disturbances, mental status alteration), anemia accompanied by fatigue, and thrombocytopenia (petechiae) [12].
The risk of bleeding and the risk of severe maternal complications vary among the underlying disease, corresponding to the high importance of the differential diagnosis. Differential diagnose should be based on the patient's personal and family history, drug history, dietary habits, physical examination and laboratory tests [3]. The diagnosis of TTP can be difficult since there are a variety of other disorders that may have the same clinical manifestations as TTP.
During pregnancy ADAMTS13 activity levels tend to be normally decreased to a 71% of normal values (41%-105%); constituting diagnostic criteria for TTP [13]. The diagnosis of TTP can be confirmed by reduced ADAMTS13 activity levels or the presence of an inhibitor or IgG antibodies [7].
An ADAMTS13 activity deficiency can also be seen in other thrombocytopenic events like preeclampsia, HELLP. Differentiating TTP form HELLP syndrome during pregnancy can be a challenge, because of the very similar manifestations (hemolysis, severe thrombocytopenia, elevated blood pressures, renal dysfunction, elevated liver enzymes, elevated LDH). Tough, TTP is characterized by a severe deficiency or absence of ADAMTS-13 activity (activity levels < 5%) whereas pre-eclampsia and HELLP syndrome usually present with activity levels of 12-43% of normal [8,13].
In the event of determining the incidence of TTP, a monocentric retrospective study (2008-2009) was conducted among pregnant women who have experienced severe thrombocytopenia (PLT < 75.000 μL) at least once. Among 8,908 deliveries, 79 women had a platelet count < 75.000 μL. The ADAMTS-13 level activity was measured in 50 women; ADAMTS13 activity was < 10% in 4, 20% in 1 and normal in 45 women [11].
Although ADAMTS13 activity level and other serological tests are available and used to confirm the diagnosis of thrombocytopenia, in some circumstances (such as low resource settings, emergent situations where delay of treatment cannot be obtained), health care providers should rely on clinical history and manifestations to discern the possible causes and manage each promptly. For example, sudden onset of anemia, thrombocytopenia and renal insufficiency are in favor of HELLP syndrome. Progressive worsening of renal function is highly suggestive of atypical HUS. Severe thrombocytopenia with neurological signs and minimal renal insufficiency are in favor of TTP [12]. HELLP syndrome and preeclampsia usually resolves within 3 days after delivery [8] (Table 3).
Table 3: Typical features in different pregnancy-associated TMAs [14]. View Table 3
TTP has also a high recurrence risk, approximately 92% in women with cTTP, 47% in aTTP and 45% in acquired pregnancy-associated TTP, whereas HELLP syndrome recurs less frequent. There is also a greater risk of preeclampsia for women with pregnancy-associated TTP. Given the increased risk of recurrence in pregnancy, women with a previous history of TTP should be monitored closely for the development of TTP [5,13,14].
The stillbirth rate in pregnancy - associated with TTP is about 40%, due to intrauterine fetal death (IUFD), spontaneous abortions and prematurity, all possibly related to placental ischemia. There may be an earlier requirement of delivery, and so the fetal prognosis depends on the gestational age at birth [7].
The early diagnosis is critical because of different management and prognosis. Treatment of an acute TMA relies on maternal factors and fetal status. The primary goal is to determine whether delivery will be beneficial, remising the TMA or whether plasma exchange (PEX) should be urgently instigated following delivery as treatment. TTP can be a fatal condition and treatment should be employed immediately under highly clinical suspicion, regardless of the ADAMTS13 activity levels. In the suspicion of TTP, PEX should be started urgently. Until PEX was established as an effective treatment, TTP was associated with high mortality rate, approximately 95%; in cases of pregnancy-related TTP, maternal survival was rare and the fetal mortality rate approached 80%. In case of cTTP and aTTP, plasma infusion and PEX respectively should be continued until delivery and postpartum [14-16].
Administration of fresh frozen plasma (FFP), glucocorticoids and rituximab can also supplement the first line's treatment. The target is the establishment of a normal platelet count >150.000 μL [14].
The management and treatment of TMA should be implemented by a multidisciplinary team, resulting, not only in better prognosis of the disease but also in less length of hospitalization and better understanding of the disease [17].
One of the main concerns in these pregnancies is the decision of performance regional anesthesia, since thrombocytopenia in pregnancy is considered a relative contraindication [10]. The lowest threshold of platelet count for a safe performance of neuraxial block is controversial in obstetric anesthesia. When it comes to evaluate the risk, one should have in mind that both absolute platelet count and platelet function may be affected when thrombocytopenia in pregnancy is present. Several studies suggest the safe placement of neuraxial block in asymptomatic parturients with a platelet count range 75,000-80,000 μL. The decision should be individualized each time, weighting the risks of general anesthesia against neuraxial complications under the clinical circumstances [18].
The anesthetic management of a parturient during general anesthesia is of great concern. A difficult airway in conjunction with the urgent nature of obstetrics and the potential risk of impacting both mother and fetus are of great apprehension among anesthesiologists. Alterations of the anatomy and physiology during pregnancy affecting obstetric airway include: Upper airway edema, breast enlargement, excessive weight gain, cephalad displacement of diaphragm, decreased functional residual capacity, increased oxygen consumption, increased risk of aspiration.
In general surgical population failed intubation occurs at a rate of 1 in 2,330. The incidence of failed intubation in the obstetrical population is approximately eight times higher than among non-obstetrical patients (1 in 280) [19]. Failed intubation, inadequate ventilation, and aspiration represent leading causes of anesthesia-associated obstetric morbidity. Therefore, neuraxial techniques, affording an opportunity to avoid airway instrumentation, are advocated for cesarean delivery [20]. In a non-emergent cesarean section, epidural or combined anesthesia can be proceeded. In emergent circumstances, one shot spinal anesthesia is preferred. Hemodynamic and respiratory compromises should be treated accordingly [19].
Performing neuraxial techniques in obstetric patients has a number of advantages, including avoiding airway instrumentation, providing effective anesthesia while minimizing maternal and neonatal sedation, allowing the parturient to experience the birth of her child, while postoperative analgesia after cesarean delivery is superior using neuraxial opioids.
Thrombocytopenia has long been considered a relative or even an absolute contraindication to neuraxial techniques due to a potential increased risk of spinal epidural haematoma. In the general obstetric population the incidence of spinal epidural haematoma is estimated to be between 1:200,000 and 1:250,000. However, the incidence in obstetric population with thrombocytopenia is unknown, and the assessment of risk still poses a challenge [20,21]. A retrospective study by Moen, et al. of neuraxial anesthetics, that included 50,000 spinal and 205,000 epidural blocks in parturients, reported only one spinal haematoma after epidural catheter removal and one after spinal blockade, but both in patients with HELLP syndrome [22]. According to a retrospective cohort study utilizing the Multicenter Peri-operative Outcomes Group (MPOG) database, the risk of epidural hematoma from neuraxial anesthesia in a parturient with a platelet count greater than 70,000 μL is exceptionally low (less than 0.2%). However, the exact risk of spinal epidural hematoma for platelet counts < 70,000 μL remains uncertain, with an upper bound of 3% for counts of 50,000-69,000 μL and 11% for counts of 0-49,000 μL [20].
A recent case report of TTP displays the case of a parturient who received spinal anesthesia with subsequent discovery of a platelet count of 7000 μL. Due to fetal distress, an urgent cesarean section was performed, without a pre-procedure platelet count. She had normal level of platelet counts earlier in the pregnancy and no history of hemorrhagic complications. No development of signs of epidural hematoma was reported despite the fact that severe thrombocytopenia existed during spinal anesthesia. After delivery, a TTP diagnosis was concluded [23].
Other professional organizations, obstetric, hematologic, oncologic, radiologic, transfusion medicine, and neurological societies, have made recommendations regarding platelet thresholds for neuraxial procedures, with the most commonly used limit for anesthetic neuraxial procedures being that of 80,000 μL.
The Scandinavian Society of Anesthesiology recommends lower thresholds for anesthetic neuraxial procedures, and they do recommend a lower threshold for single-shot spinal compared to epidural. In fact, there has been a lot of discussion whether the spinal has a lower risk of spinal epidural hematoma compared with epidural procedure. This hypothesis is, plausible, based on the different types of needle most commonly been used for each technique in obstetric patients. A smaller gauge (25-29G) and "pencil point" needle tip used for spinal; larger (17-18G) "cutting" needle tip used for epidural procedures, where a catheter is also placed. However, this hypothesis cannot be supported; the literature in patients with thrombocytopenia is sparse [21].
Another significant challenge is the use of anticoagulant and anti-thrombotic drugs that can increase the risk of spinal hematoma formation, especially in pregnant patients, as neuraxial blockade is commonly used to provide labor analgesia and anesthesia for cesarean delivery.
Aspirin irreversible inhibits cyclooxygenase-1 (COX-1), required for the formation of thromboxane A2, that is necessary for platelet aggregation [21,24]. A low dose of 30 mg per day is sufficient to completely block thromboxane A2 production in 5-7 days, compared with a dose of 100 mg that can suppress almost completely COX-1 at 24 hours. The increasing dose reduces the time needed for a maximum inhibition. Normal hemostatic function has been shown in vivo with 20-30% of platelet normal COX-1 activity. Depending on the dose of aspirin, COX-2 inhibition can be produced, resulting in thrombogenic effects. In vivo, the functional defect for prevention of atherothrombotic vascular disease of a single oral dose of 75-81 mg per day is similar with that of 300-325 mg per day. It has been described an overall incidence of spinal hematoma ranging from 1:20,000 to 1:58,000 neuraxial blockades, mainly related to epidural anesthesia rather than spinal [24].
According to ASRA latest guidelines, there is no recommendation to withhold aspirin to perform a neuraxial procedure, since they do not add a significant risk to the development of spinal epidural hematoma. However, no concerns regarding dosing and/or timing of administration are being raised. In addition, it is not clear whether the risk of spinal epidural hematoma is increased by aspirin in the setting of thrombocytopenia [21,24]. ESA also recommends performing neuraxial blockade without any precise to doses.
Aspirin significantly increases the risk of spinal epidural haematoma after neuraxial blockade when administered in conjunction with anticoagulant therapy, especially heparin. Thromboprophylaxis with heparin at patients on chronic aspirin therapy increases the risk of spinal epidural haematoma by seven times. A traumatic neuraxial puncture can also increase the risk of spinal haematoma from 11-fold without concomitant heparin therapy, up to 35-fold if heparin is used. In general, neuraxial blockade could be performed at least 10-12 hours after thromboprophylactic dose, and 24 hours after full therapeutic dose of low molecular weight heparin (LMWH). However, ESA and ASRA guidelines recommend a cautionary, but yet unspecified, approach in the presence of aspirin [24].
Our patient was receiving 100 mg of acetylsalicylic acid that was stopped five days before the scheduled cesarean section. In the majority of cases, no special precautions are required for performing neuraxial anesthesia in patients receiving acetylsalicylic acid (75 to 300 mg) alone [22].
Taking into consideration the reported literature and "safe" laboratory profile of the parturient (stable variation of PLT count during 33-37 week of gestation), we decided to perform a single-shot spinal anesthesia and to avoid epidural catheter placement because we couldn't anticipate the number of platelets after surgery.
The decision to proceed with neuraxial analgesia or anesthesia in parturients with thrombocytopenia, receiving anticoagulation drugs requires careful consideration, to be individualized and decision making should be based on careful risk-benefit analysis. Factors to be considered are not limited to comorbidities but also obstetric risk factors, airway examination, availability of airway equipment, risks of general anesthesia, and patient preference. Emphasis should be given on the evaluation of platelet counts before and during pregnancy, bleeding history and possible underlying disorders of hemostasis.
Obstetricians, Anesthesiologists and Hematologists should work as a multidisciplinary team to optimize the outcome of these clinical cases.
The authors declare that there is no conflict of interest regarding the publication of this paper.