A major problem in public health in developing countries is hospital-acquired infections for which hand hygiene of healthcare workers features as a critical preventive mechanism. In this study we seek through systematic review of extant literatures to identify the most effective hand hygiene approach practiced by healthcare workers in developing countries that leads to hospital-associated infection and antimicrobial reduction.
In order to identify the hand hygiene method that is effective and appropriate in reducing both hospital-acquired infections and antimicrobial infection, we used a three-step search strategy to search to identify studies in which hand hygiene in the following libraries: Cochrane Registry, Medline, PsycINFO, JBI, Medline, EMBASE and CINAHL in The University of Nottingham e-library. We also used the System for Information on Grey Literature (SIGLE) to identify articles that contains our inclusion criteria. The Cochrane Collaboration Public Health Group data extraction tool was also used to extract the reviewed articles based on: author/s name, publication date, study design, setting, study size, study methods, hand hygiene interventions type, and study conclusion.
Of the 1,413 articles retrieved for this review, only eight were eligible for inclusion. Three studies compared the efficacy of hand hygiene products on hospital- associated infection reduction. Six studies used the WHO multimodal hand hygiene strategy; of which one used positive deviance to improve hand hygiene compliance amongst healthcare workers.
Our review suggests the WHO multimodal hand hygiene strategy alongside with alcohol hand rub use can result in effective HAI and antimicrobial reduction as well as lead to an increase HH compliance.
Multimodal, Hygiene, Infection, Healthcare
Hospital-acquired infections (HAIs) also called nosocomial infections affects patients in healthcare [1,2]. The National Institute for Health and Care Excellence guidelines for HAI control states that HAI is acquired during healthcare interventions [3]. HAIs can worsen disease conditions [4] and is increasing in low-income countries [5]. The recent surge in HAI is associated with the rising prevalence of multidrug resistance infections [6] and emergence of infectious diseases [7]. Infectious disease mortality accounts for 26% of years of life lost (YLL) globally [8] and developing countries account for 88% of communicable diseases YLL [9,10]. Fifteen percent of HAIs cases occur in developing countries [11]. HAI global mortality and morbidity makes finding a solution a global concern [12]. The spread of HAIs in developing countries is due to factors relating to the training and qualification HCWs [13]. The identification of appropriate control interventions for HAIs is an urgent public health concern [14]. The frequency of using HH is crucial in preventing and controlling HAIs [15] but without compliance HH is unsuccessful [16]. The WHO World Alliance for Patient Safety's 'Clean Care is Safer Care' campaign launched in 2015 aim at increasing use of HH strategies [17]. Compliance to HH is still a global concern despite HH being an important HAI control strategy [18-22]. More evidence of HH impact in reducing HAI in developing countries is required [23] though few research have assessed HH effectiveness among HCWs in developing countries [24]. A South African cluster randomised controlled trial (RCT) showed no statistical or clinical significance associating HH products use and HAI reduction [25]. Karabay, et al. reported a statistical and clinical significant association between efficiency and alcohol hand rubbing among HCWs [26]. Salamati, Poursharifi, and Akbar Rahbarimanesh showed a significant association when education was combined with motivational interview to improve HH among HCWs [27]. The Hawthorne effect which affects HH effectiveness and validity can produce varying findings during overt and covert observational studies. Kovacs-Litman, Wong and Shojania reported varying HH compliance due to Hawthorne effect among physicians; 84% in overt observation vs. 50% in covert observation [18].
In this systematic review we are set to identify the most effective and appropriate HH method used by HCWs in developing countries that is associated with HAI and antimicrobial reduction.
We reviewed extant literatures on HH interventions by HCWs in healthcare settings in developing countries published between 2005 and 2016. Study participants were 18 years and above, and residents of developing countries. Two reviewers (IVM and JT) assessed the selected data based on the inclusion criteria. We keyed search terms and/their alternatives for literatures using the review Patient problem, Intervention Comparison and Outcome(PICO) [28] to identify studies in which HH interventions were used. A three-step search strategy was used to search the following libraries: Cochrane Registry [29], Johanna Briggs Institute (JBI) [30], Excerpta Medica database (EMBASE) [31] and the Cumulative Index to Nursing and Allied Health Literature (CINHAHL) [31]. The sentence structure of online search filters (SIGN) was used to identify the required articles while the System for Information on Grey Literature (SIGLE) [32] was employed if the reviewed article heading contains the inclusion criteria.
The Cochrane Collaboration Public Health Group data extraction tool was used to extract the reviewed articles based on: (a) Author/s name; (b) Date of publication; (c) Study design; (d) Setting; (e) Study size; (f) Methods; (g) Interventions; and (h) Conclusion [29]. Critical appraisal was used to determine the methodological quality of the reviewed articles. Garg, Hackman and Tonelli have argued that a systematic review reliability and quality depends on the quality of the reviewed articles [30]. We also used the Cochrane Collaboration's PEDro scale [29] and the Effective Practice and Organisation of Care (EPOC-RoB) Tool [31] to review RCTs and Non-RCTs studies respectively in this review. A Cochrane's Collaboration GRADE tool was further employed as a guide for evidence estimation [31] while the Preferred Reporting Items for SRs and Meta-analysis (PRISMA-P) flow diagram [31] was used to assess for study bias and appropriateness. We further used the Cochrane Collaborations Risk of Bias Tool was used to summarise the risk assessment for 5 studies in this review.
The University of Nottingham, UK Institutional Review Board provided ethical clearance and approved this study.
We commenced the initial search for articles relating to the review topic on the 12th March 2016 to the final search date of 7th July 2016 but excluded large number of unrelated articles and limiters based on dates (2005-2016), language and geographical region (Table 1).
Table 1: Summary of literature search history. View Table 1
A summary of literature search history using search limiters such as Article title, published dates and geographic regions.
We retrieved 1,413 articles; 1,353 from databases and 60 from other sources. 1,405/1,413 (99.4%) were excluded following titles, abstracts and contents assessment. The 8/1,413 (0.6%) publications reviewed are: Li, et al. [33], Allegranzi, et al. [34], Sharma, et al. [35], Chen, et al. [36], Marra, et al. [37], Kampiatu and Cozean [38], Rosenthal, et al. [39], and Patel, et al. [40].
EPOC-RoB was used to summarise the methodological quality and risk for non-randomised controlled trials (Table 2).
Table 2: EPOC Tool for assessment of methodical quality of Non-RCTS. View Table 2
Eight studies based on sample selection randomisation, evidence of attrition bias, and evidence of allocation concealment and selection bias were reviewed.
The eight studies reviewed included four studies that used randomised sample selection (Kampiatu, et al. [38], Li, et al. [33], Marra, et al. [37], and Sharma, et al. [35]), and 4 studies (Allegranzi, et al. [34], Chen, et al. [36], Patel, et al. [40], Rosenthal, et al. [39]) that made use of evidence of attrition bias and allocation concealment as well as selection bias (Table 3).
Table 3: Appropriateness analysis of reviewed literatures. View Table 3
Fifty percent of the literature reviewed reported procedures to identify and eliminate study bias.
The Cochrane Collaborations Risk of Bias Tool was used to summarise the risk assessment for 5 studies in this review (Figure 1).
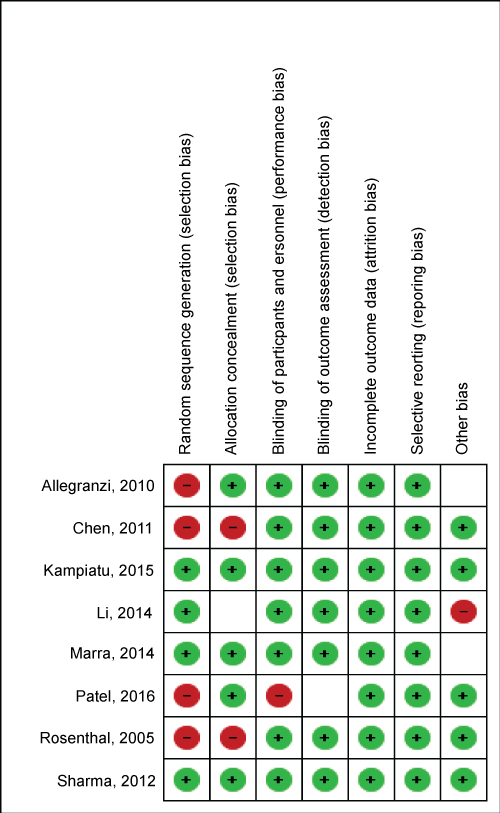 Figure 1: Cochrane risk of bias assessment tool for the studies reviewed. View Figure 1
Figure 1: Cochrane risk of bias assessment tool for the studies reviewed. View Figure 1
Cochrane risk of bias assessment tool for the eight studies reviewed alongside with review terms including random sequencing, allocation concealment, participants blinding, outcome assessment blinding, attribution and reporting biases.
The comparability of study designs and the cost-effectiveness of HH products were considered during the review process. Higgins, et al. have pointed out the significance of comparability of studies in terms of effect size [41]. Sharma, et al. [35] and Li, et al. [33] recorded reduced colony-forming units (CFU) using different methods. Kampiatu, et al. [38], Sharma, et al. [35] and Chen, et al. [36] showed the effectiveness of HH products in reducing HAIs. Kampiatu, et al. [38] demonstrated the effectiveness of alcohol hand rub in reducing HAIs in healthcare settings in developing country from 23.1% to 0% (p < 0.005). Sharma, et al. [35] also demonstrated the effective use of alcohol in reducing CFU [OR 3.2 (95% CI 1.9, 5.3)]. Chen, et al. [36] reported a correlation (r = 0.9399, p = 0.001) between increased (99.9%) use of alcohol hand rub and HAIs trends (Table 4).
Table 4: Assessment of articles reviewed for study outcomes. View Table 4
Seventy five percent of the literature reviewed used WHO multimodal HH strategy while 87.5% reported HAI reduction as a result of HH strategy.
Allegranzi, et al. [34] and Rosenthal, et al. [39] reported the use of WHO multimodal strategy, alcohol hand rub and health education leads to HAI reduction and an increase in the use of alcohol hand rub as disinfectant. Patel, et al. [40] and Marra, et al. [37] demonstrated WHO multimodal HH strategy effectiveness in improving HH compliance among HCWs. Patel, et al. [40] reported a 13% and 6% increase in intervention compliance for 2014 and 2015 respectively (p < 0.05). Marra, et al. [37] study recorded an increase (96.1% increase, p < 0.05) in alcohol hand rub as disinfectant, reduction (2.9% reduction, p < 0.05) in HAIs and an improvement in compliance (15.9% compliance rate, p < 0.05). Li, et al. [33] reported a 97.2% CFU reduction when comparing the efficacy and time effectiveness of using alcohol hand rub (p < 0.001).
Our study produced evidence to demonstrate the significance of HH compliance before and after patient contact in HAI prevention. HH compliance among HCWs is a major problem especially in developing countries [24].
Three key concepts emerged from this study: Effectiveness of HH methods for HAI reduction, HH compliance determined by the HAI and CFU reduction and the comparative efficacy of HH products alongside with the WHO multimodal HH strategies in reducing HAI.
Kampiatu, et al. [38] reported HAI reduction from 23.1% to 0% (p < 0.005) in a study that assessed the effectiveness of persistent and sustained antimicrobial use on handwashing alone. We believe that the persistent effect (compliance) of antimicrobial product affected the outcome in the Kampiatu, et al. by acting as a confounder. Kampiatu, et al. [38] did not demonstrate the residual effect of antimicrobial product use in their study though their findings were consistent with the Czerwinski, Cozean and Cozean trial which reported a 100% antimicrobial reduction due to alcohol-based antiseptic use [41]. Also, an RCT by Chow, Ara and Chan demonstrated the efficacy and time effectiveness of alcohol hand rub [42].
Sharma, et al. [35] in their comparative analysis reported a statistically and clinically significant percentage reduction in CFU (< 50% reduction, p < 0.001). The Sharma, et al. [35] findings supports the study by Won, et al. which associated HH intervention using different antimicrobial products to HAI reduction (p = 0.003) [43]. The strength of the Sharma, et al. study was the evaluation of actual HH intervention during clinical activities. This method differs from the artificial method used in the laboratory-based randomized crossover trial by Gnatta, et al. [44]. Ling, et al. [45] and Chen, et al. [36] in a hospital-wide before-and-after study which superimposed HH intervention on an infection control programme also recorded a significant HAI reduction.
Rosenthal, et al. study observed a sustained improvement in HH compliance and a significant reduction in HAIs (RR = 0.59; 95% CI: 0.46~0.74, p < 0.0001) [39]. The Rosenthal, et al. study shows that HH intervention user enjoys a protective effect. A cost-benefit analysis which parallels the Rosenthal, et al. study shows significant cost difference in alcohol hand rub [39].
The study by Allegranzi revealed an improvement (OR, 2.50; 95% CI, 1.8-3.5) in HAIs reduction across all medical specialties with enhanced knowledge increasing compliance by 28.1% [39]. Though the Allegranzi suggested that HH intervention should be multimodal, the quality of the study for such a decision is questionable since it was conducted with no proper control group to validate their claims. Patel, et al. showed that compliance to HH using the WHO multimodal strategy could lead to a 42% increase in CFU reduction in the subsequent year [41]. Chen, et al. however demonstrated that incentive ($160.00) can increase performance using HH compliance as a quality indicator [40]. The Chen, et al. study however lacks detailed rationale for the variation in the effect of HH intervention on HAI.
Yardly, et al. suggests that encouraging HH compliance could lead to the over-estimation of self-reported HH adherence [46]. Patel, et al. reported a 42% increase in CFU reduction following the HH-related intervention [41] which is lower than that of Randle, Firth and Natalie (74% increase) [47]. The low compliance rate in the Patel, et al. trial could be due to selection bias in the choice of intervention wards which may have peer influence that could have impinged negatively on the staffs.
Peer influence had a significant positive effect on HCWs in the study by Marra, et al. which recorded a 55.1% compliance improvement [37]. Moongtui reported a 74% improved compliance among HCWs due to peer feedback [48].
Our review suggests that HH strategies including alcohol hand rub use can result in increased compliance and HAI reduction. Six out of 8 studies reviewed (Kampiatu, et al. [35]; Li, et al. [36]; Marra, et al. [37], Sharma, et al. [38]; Chen, et al. [40], and Allegranzi, et al. [39]) shows a statistical and clinical significance for alcohol hand rub with positive cost-benefit ratio (23.7 with 3% discount rate). The Allegranzi, et al. [45] study produced similar results though with low applicability to low-resource countries hence the recommendation for the use of locally produced alcohol hand rubs in resource poor countries to cut cost [45].
One limitation of this systematic review is the methodological heterogeneity of the studies reviewed which did not allow the quantification of the relative efficacy of the interventions and outcome.
Our review is consistent with that of Picheansathian which reported the effectiveness of alcohol-based hand rub in controlling HAIs [49]. We discovered that alcohol hand rub is the most effective HH intervention among HCWs in developing countries. We recommend alcohol hand rub use alongside with the WHO multimodal strategy to promote HH concordance among HCWs. We are also recommending further RCT study to scientifically analyse the effectiveness of HH interventions.
The Institutional Review Board of the University of Nottingham, UK approved this study and provided ethical clearance for conducting this study.
Not applicable.
No dataset was used for this study. This is a systematic review that reviewed previously published works.
All authors declared they have no competing interest.
No part of this study received funding or compensation whatsoever during its conception, execution or for publication.
IVM conceived and designed this study as well as organized the conduct of this research in the research field. IVM, SM and JBK reviewed literatures. IVM and JBK drafted the manuscript. JBK critically reviewed and revised the manuscript.
Our sincere thanks to the supervisors and lecturers at the Nursing Department of The University of Nottingham, UK.
Not applicable.